Abstract
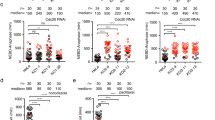
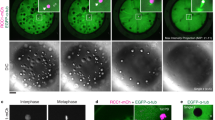
Mad2 has a key role in the spindle-assembly checkpoint (SAC) — the mechanism delaying anaphase onset until all chromosomes correctly attach to the spindle. Here, we show that unlike every other reported case of SAC inactivation in metazoans, mad2-null Drosophila are viable and fertile, and their cells almost always divide correctly despite having no SAC and an accelerated 'clock', which is caused by premature degradation of cyclin B. Mitosis in Drosophila does not need the SAC because correct chromosome attachment is achieved very rapidly, before even the cell lacking Mad2 can initiate anaphase. Experimentally reducing spindle-assembly efficiency renders the cells Mad2-dependent. In fact, the robustness of the SAC may generally mask minor mitotic defects of mutations affecting spindle function. The reported lethality of other Drosophila SAC mutations may be explained by their multifunctionality, and thus the 'checkpoint' phenotypes previously ascribed to these mutations should be considered the consequence of eliminating both the checkpoint and a second mitotic function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Musacchio, A. & Hardwick, K. G. The spindle checkpoint: structural insights into dynamic signalling. Nature Rev. Mol. Cell Biol. 3, 731–741 (2002).
Basu, J. et al. Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila. J. Cell Biol. 146, 13–28 (1999).
Dobles, M., Liberal, V., Scott, M. L., Benezra, R. & Sorger, P. K. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101, 635–645 (2000).
Kalitsis, P., Earle, E., Fowler, K. J. & Choo, K. H. Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes Dev. 14, 2277–2782 (2000).
Kitagawa, R. & Rose, A. M. Components of the spindle-assembly checkpoint are essential in Caenorhabditis elegans. Nature Cell Biol. 1, 514–521 (1999).
Sironi, L. et al. Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint. EMBO J. 20, 6371–6382 (2001).
May, K. M. & Hardwick, K. G. The spindle checkpoint. J. Cell Sci. 119, 4139–4142 (2006).
Buffin, E., Lefebvre, C., Huang, J., Gagou, M. E. & Karess, R. E. Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex. Curr. Biol. 15, 856–861 (2005).
Kops, G. J. et al. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 169, 49–60 (2005).
Karess, R. Rod–Zw10–Zwilch: a key player in the spindle checkpoint. Trends Cell Biol. 15, 386–392 (2005).
Burds, A. A., Lutum, A. S. & Sorger, P. K. Generating chromosome instability through the simultaneous deletion of Mad2 and p53. Proc. Natl Acad. Sci. USA 102, 11296–11301 (2005).
Williams, B., Karr, T., Montgomery, J. & Goldberg, M. The Drosophila l(1)zw10 gene product, required for accurate mitotic chromosome segregation is redistributed at anaphase onset. J. Cell Biol. 118, 759–773 (1992).
Lopes, C. S., Sampaio, P., Williams, B., Goldberg, M. & Sunkel, C. E. The Drosophila Bub3 protein is required for the mitotic checkpoint and for normal accumulation of cyclins during G2 and early stages of mitosis. J. Cell Sci. 118, 187–198 (2005).
Karess, R. & Glover, D. rough deal: A gene required for proper mitotic segregation in Drosophila. J. Cell Biol. 109, 2951–2961 (1989).
Basto, R., Gomes, R. & Karess, R. E. Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila. Nature Cell Biol. 2, 939–943 (2000).
Pfleger, C. M., Lee, E. & Kirschner, M. W. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase- promoting complex. Genes Dev. 15, 2396–2407 (2001).
Chen, J. & Fang, G. MAD2B is an inhibitor of the anaphase-promoting complex. Genes Dev. 15, 1765–1770 (2001).
Meyer, V., Oliver, B. & Pauli, D. Multiple developmentary requirements of noisette, the Drosophila homolog of the U2 snRNP-associated polypeptide SP3a60. Mol. Cell Biol. 18, 1835–1843 (1998).
Siller, K. H., Serr, M., Steward, R., Hays, T. S. & Doe, C. Q. Live imaging of Drosophila brain neuroblasts reveals a aole for Lis1/Dynactin in spindle assembly and mitotic checkpoint control. Mol. Biol Cell. 16, 5127–5140 (2005).
Savoian, M. S. & Rieder, C. L. Mitosis in primary cultures of Drosophila melanogaster larval neuroblasts. J. Cell Sci. 115, 3061–3072 (2002).
Meraldi, P., Draviam, V. M. & Sorger, P. K. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell 7, 45–60 (2004).
Liu, S. T. et al. Human CENP-I specifies localization of CENP-F, MAD1 and MAD2 to kinetochores and is essential for mitosis. Nature Cell Biol. 5, 341–345 (2003).
Sudakin, V., Chan, G. K. & Yen, T. J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925–936. (2001).
Huang, J.-Y. & Raff, J. W. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J. 18, 2184–2195 (1999).
Raff, J. W., Jeffers, K. & Huang, J. Y. The roles of Fzy/Cdc20 and Fzr/Cdh1 in regulating the destruction of cyclin B in space and time. J. Cell Biol. 157, 1139–1149 (2002).
Clute, P. & Pines, J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nature Cell Biol. 1, 82–87 (1999).
Hagting, A. et al. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157, 1125–1137 (2002).
Megraw, T. L., Kao, L. R. & Kaufman, T. C. Zygotic development without functional mitotic centrosomes. Curr. Biol. 11, 116–120 (2001).
Starr, D. A., Williams, B. C., Hays, T. S. & Goldberg, M. L. ZW10 helps recruit Dynactin and Dynein to the kinetochore. J. Cell Biol. 142, 763–774 (1998).
Lampson, M. A. & Kapoor, T. M. The human mitotic checkpoint protein BubR1 regulates chromosome–spindle attachments. Nature Cell Biol. 7, 93–98 (2005).
Meraldi, P. & Sorger, P. K. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J. 24, 1621–1633 (2005).
Montagne, J. et al. Drosophila S6 kinase: a regulator of cell size. Science 285, 2126–2129 (1999).
Acknowledgements
We thank: J. Montagne and D. Pauli for stocks of Ds6k and noisette aberrations, respectively; J. Raff for GFP–cyclin B flies; G. Rogers for his unpublished Mad2 antibody; and H. Quesneville and M.-H. Mucchielli for help with statistics. This work was supported by grants from the Centre National de la Recherche Scientifique (CNRS) to R.K., the Association pour la Recherche sur le Cancer (ARC) to R.K. and E.B., La Ligue Nationale Contre le Cancer to R.K., and Le Ministère de l'Education Nationale de la Recherche et de la Technologie to E.B. and D.E.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figure S1, Supplementary Tables S1, S2 and Supplementary Materials (PDF 517 kb)
Supplementary Information
Supplementary Movie 1 (MOV 1212 kb)
Supplementary Information
Supplementary Movie 2 (MOV 986 kb)
Supplementary Information
Supplementary Movie 3 (MOV 2394 kb)
Supplementary Information
Supplementary Movie 4 (MOV 2461 kb)
Supplementary Information
Supplementary Movie 5 (MOV 612 kb)
Supplementary Information
Supplementary Movie 6 (MOV 675 kb)
Supplementary Information
Supplementary Movie 7 (MOV 393 kb)
Supplementary Information
Supplementary Movie 8 (MOV 662 kb)
Rights and permissions
About this article
Cite this article
Buffin, E., Emre, D. & Karess, R. Flies without a spindle checkpoint. Nat Cell Biol 9, 565–572 (2007). https://doi.org/10.1038/ncb1570
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1570
This article is cited by
-
Aneuploidy is Linked to Neurological Phenotypes Through Oxidative Stress
Journal of Molecular Neuroscience (2024)
-
Centrosomes are multifunctional regulators of genome stability
Chromosome Research (2016)
-
How oocytes try to get it right: spindle checkpoint control in meiosis
Chromosoma (2016)
-
Centrosomes in spindle organization and chromosome segregation: a mechanistic view
Chromosome Research (2016)
-
Spindle assembly checkpoint inactivation fails to suppress neuroblast tumour formation in aurA mutant Drosophila
Nature Communications (2015)