Abstract
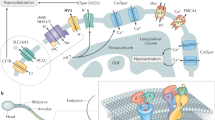
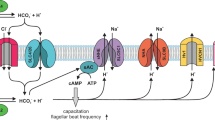
Cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-activated chloride channel expressed in a wide variety of epithelial cells, mutations of which are responsible for the hallmark defective chloride secretion observed in cystic fibrosis (CF). Although CFTR has been implicated in bicarbonate secretion, its ability to directly mediate bicarbonate secretion of any physiological significance has not been shown. We demonstrate here that endometrial epithelial cells possess a CFTR-mediated bicarbonate transport mechanism. Co-culture of sperm with endometrial cells treated with antisense oligonucleotide against CFTR, or with bicarbonate secretion-defective CF epithelial cells, resulted in lower sperm capacitation and egg-fertilizing ability. These results are consistent with a critical role of CFTR in controlling uterine bicarbonate secretion and the fertilizing capacity of sperm, providing a link between defective CFTR and lower female fertility in CF.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Quinton, P.M. Physiological basis of cystic fibrosis: a historical perspective. Physiol. Rev. 79, S3–S22 (1999).
Quinton, P.M. The neglected ion: HCO3−. Nature Med. 7, 292–293 (2001).
Choi, J.Y. et al. Aberrant CFTR-dependent HCO3− transport in mutations associated with cystic fibrosis. Nature 410, 94–97 (2001).
Poulsen, J.H., Fischer, H., Illek, B. & Machen, T.E. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl Acad. Sci. USA 91, 5340–5344 (1994).
Illek, B., Yankaskas, J.R. & Machen, T.E. cAMP and genistein stimulate HCO3− conductance through CFTR in human airway epithelia. Am. J. Physiol. 272, L752–L761 (1997).
Hug, M.J., Tamada, T. & Bridges, R.J. CFTR and bicarbonate secretion by epithelial cells. News Physiol. Sci. 18, 38–42 (2003).
Kopito, L.E., Kosasky, H.J. & Shwachman, H. Water and electrolytes in cervical mucus from patients with cystic fibrosis. Fertil. Steril. 24, 512–516 (1973).
Oppenheimer, E.A., Case, A.L., Esterly, J.R. & Rothberg, R.M. Cervical mucus in cystic fibrosis: a possible cause of infertility. Am. J. Obstet. Gynecol. 108, 673–674 (1970).
Trezise, A.E. & Buchwald, M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature 353, 434–437 (1991).
Chan, L.N. et al. Distribution and regulation of ion channels in murine female reproductive tract. J. Membrane Biol. 185, 165–176 (2002).
Tizzano, E.F., Silver, M.M., Chitayat, D., Benichou, J.C. & Buchwald, M. Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues. Clues for the infertility in patients with cystic fibrosis. Am. J. Pathol. 144, 906–914 (1994).
Vishwakarma, P. The pH and bicarbonate-ion content of the oviduct and uterine fluids. Fertil. Steril. 13, 481–485 (1962).
Shi, Q.X. & Roldan, E.R. Bicarbonate/CO2 is not required for zona pellucida- or progesterone-induced acrosomal exocytosis of mouse spermatozoa but is essential for capacitation. Biol. Reprod. 52, 540–546 (1995).
Visconti, P.E. et al. The molecular basis of sperm capacitation. J. Androl. 19, 242–248 (1998).
Chen, Y. et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625–628 (2000).
Wang, X.F. et al. The involvement of Na+-HCO3− cotransporter in mediating cAMP-dependent HCO3− secretion by mouse endometrial epithelium. Biol. Reprod. 66, 1846–1852 (2002).
Chan, H.C. et al. Electrogenic ion transport in the mouse endometrium: functional aspects of the cultured epithelium. Biochim. Biophys. Acta 1356, 140–148 (1997).
Sheppard, D.N. & Welsh, M.J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J. Gen. Physiol. 100, 573–591 (1992).
Schultz, B.D., Singh, A.K., Devor, D.C. & Bridges, R.J. Pharmacology of CFTR chloride channel activity. Physiol. Rev. 79, S109–S144 (1999).
Ward, C.R. & Storey, B.T. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev. Biol. 104, 287–296 (1984).
Madden, M.E. & Sarras, M.P. Jr. Morphological and biochemical characterization of a human pancreatic ductal cell line (PANC-1). Pancreas 3, 512–528 (1988).
Cheng, H.S. et al. Concurrent and independent HCO3− and Cl− secretion in a human pancreatic duct cell line (CAPAN-1). J. Membr. Biol. 164, 155–167 (1998).
Drumm, M.L. et al. Correction of the cystic fibrosis defect in vitro by retrovirus- mediated gene transfer. Cell 62, 1227–1233 (1990).
Epelboin, S. et al. Management of assisted reproductive technologies in women with cystic fibrosis. Fertil. Steril. 76, 1280–1281 (2001).
Fraser, L.R. Mouse sperm capacitation assessed by kinetics and morphology of fertilization in vitro. J. Reprod. Fertil. 69, 419–428 (1983).
Acknowledgements
The work was supported by the Strategic Program of the Chinese University of Hong Kong, Distinguished Young Investigator Fund by National Science Foundation of China (to H.C.C.) and National 973 Project of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wang, X., Zhou, C., Shi, Q. et al. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat Cell Biol 5, 902–906 (2003). https://doi.org/10.1038/ncb1047
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1047
This article is cited by
-
Identification and selection of healthy spermatozoa in heterozygous carriers of the Phe508del-variant of the CFTR-gene in assisted reproduction
Scientific Reports (2022)
-
The Potential Relationship Between Different Human Female Reproductive Disorders and Sperm Quality in Female Genital Tract
Reproductive Sciences (2022)
-
In vivo measurement of pH and CO2 levels in the uterus of sows through the estrous cycle and after insemination
Scientific Reports (2021)
-
Bicarbonate permeation through anion channels: its role in health and disease
Pflügers Archiv - European Journal of Physiology (2020)
-
Manipulation of bicarbonate concentration in sperm capacitation media improves in vitro fertilisation output in porcine species
Journal of Animal Science and Biotechnology (2019)