Abstract
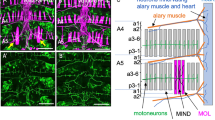
The Drosophila fruitless (fru) gene product Fru has been postulated to be a neural sex-determination factor that directs the development of at least two male-specific characteristics, namely courtship behaviour and formation of the muscle of Lawrence (MOL). The fru gene encodes a putative transcription factor with a BTB domain and two zinc-finger motifs, and with consensus Tra-binding sequences. The binding of Tra to these sequences results in sex-specific alternative splicing of the fru mRNA, leading to production of the ‘male-type’ or ‘female-type’ Fru protein. We show here that the Fru protein is not detected in the female central nervous system (CNS), despite the similar level of expression of fru mRNA in both male and female CNS. As ectopic expression of both the ‘male-type’ (with the sequence for the amino-terminal extension) and ‘female-type’ (without the sequence for the amino-terminal extension) fru cDNA can induce formation of the MOL in females, the presence or absence of the Fru protein, and not its sex-specific structure, seems to be responsible for the sexually dimorphic actions of the fru gene.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Marin, I. & Baker, B. S. The evolutionary dynamics of sex determination. Science 281, 1990– 1994 (1998).
Yamamoto, D., Fujitani, K., Usui, K., Ito, H. & Nakano, Y. From behavior to development: genes for sexual behavior define the neuronal sexual switch in Drosophila. Mech. Dev. 73, 135–146 (1998).
Taylor, B. J. Differentiation of a male-specific muscle in Drosophila melanogaster does not require the sex-determining genes doublesex or intersex. Genetics 132, 179–191 ( 1992).
Villella, A. & Hall, J. C. Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics 143, 331–344 (1996).
Ito, H., Fujitani, K., Usui, K., Shimizu-Nishikawa, K., Tanaka, S. & Yamamoto, D. Sexual orientation in Drosophila is alternated by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl Acad. Sci. USA 93, 9687~9692 (1996).
Ryner, L. C. et al. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87, 1079 –1089 (1996).
Waterbury, J. A., Jackson, L. L. & Schedl, P. Analysis of the doublesex female protein in Drosophila melanogaster: Role in sexual differentiation and behavior and dependence on intersex. Genetics 152, 1653– 1667 (1999).
Heinrichs, V., Ryner, L. C. & Baker, B. S. Regulation of sex-specific selection of fruitless 5 ’ splice sites by transformer and transformer-2. Mol. Cell. Biol. 18, 450–458 (1998).
Goodwin, S. F. et al. Aberrant splicing and altered spatial expression patterns in fruitless mutants of Drosophila melanogaster. Genetics 154, 725–745 (2000).
Kaufman, R. J., Murtha, P. & Davies, M. V. Translation efficiency of polycistronic mRNAs and their utilization to express heterologous genes in mammalian cells. EMBO J. 6, 187–193 ( 1987).
Parkers, T. L., Elia, A. J., Dickinson, D., Hilliker, A. J., Phillips, J. P. & Boulianne, G. L. Extension of Drosophila lifespan by overexpression of human SOD1 in motoneurons. Nature Genet. 19, 171–174 (1998).
Lawrence, P. A. & Johnston, P. The genetic specification of pattern in a Drosophila muscle. Cell 36, 775–782 (1984).
Lawrence, P. A. & Johnston, P. The muscle pattern of a segment of Drosophila may be determined by neurons and not by contributing myoblasts. Cell 45, 505– 513 (1986).
Du, C., McGuffin, M. E., Dauwalder, B. L., Robinow, L. & Mattox, W. Protein phosphorylation plays an essential role in the regulation of alternative splicing and sex determination in Drosophila. Mol. Cell 2, 741– 750 (1998).
Charlesworth, B. The evolution of chromosomal sex determination and dosage compensation. Curr. Biol. 6, 149–162 ( 1996).
Kelley, R. L., Wang, J., Bell, L. & Kuroda, M. I. Sex lethal controls dosage compensation in Drosophila by a nonsplicing mechanism. Nature 387, 195–199 (1997).
Bashaw, G. J. & Baker, B. S. The regulation of the Drosophila msl-2 gene reveals a function for Sex-lethal in translational control. Cell 89, 789–798 ( 1997).
Merendino, L., Guth, S., Bilbao, D., Martínez, C. & Valcárcel, J. Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature 402, 838–841 (1999).
Bopp, D., Calhoun, G., Horabin, J. I., Samuels, M. & Schedl, P. Sex-specific control of Sex-lethal is a concerved mechanism for sex determination in the genus Drosophila . Development 122, 971–982(1996).
Ito, K., Sass, H., Urban, J., Hofbauer, A. & Schneuwly, S. GAL 4-responsive UAS-tau as a tool for studying the anatomy and development of the Drosophila central nervous system. Cell Tissue Res. 290, 1–10 (1997).
Baba, K., Takeshita, A., Majima, K., Ueda, R., Kondo, S., Juni, N. & Yamamoto, D. The Drosophila Bruton’s tyrosine kinase (Btk) homolog is required for adult survival and male genital formation. Mol. Cell. Biol. 19, 4405–4413 (1999).
Lukacsovich, T., Asztalos, Z., Juni, N., Awano, W. & Yamamoto, D. The Drosophila melanogaster 60A chromosomal division is extremely dense with functional genes: their sequences, genomic organization, and expression. Genomics 57, 43– 56 (1999).
Inoue, K., Hoshijima, K., Higuchi, I., Sakamoto, H. & Shimura, Y. Binding of the Drosophila transformer and transformer-2 proteins to the regulatory elements of doublesex primary transcript for sex-specific RNA processing. Proc. Natl Acad. Sci. USA 89, 8092–8096 ( 1992).
Acknowledgements
We thank S. Niwa for her assistance with the preliminary experiments, S. C. Fujita, J. C. Hall, O. Burtis, S. Robinow, K. Inoue, K.-i. Kimura, and J. P. Phillips for the antibodies, fly stocks, plasmids and cDNAs, and S. Kondo and Y. Kai for secretarial assistance. This study was supported in part by Special Coordination Funds for Promoting Science and Technology from the Science and Technology Agency of Japan (to D.Y.), and Waseda University Grant No. 99 A-885.
Correspondence and requests for materials should be addressed to D.Y. The nucleotide sequences described here have been deposited at GenBank under following the accession numbers:
AF220176 (fru-A-female type), AF221077 (fru-A-male type), AF220178 (fru-B-exon 8), AF220179 (fru-C-exon 8), AF220180 ( fru-D-exon 7 to 3′ end) and AF220181 (fru-E-exon 8).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Usui-Aoki, K., Ito, H., Ui-Tei, K. et al. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat Cell Biol 2, 500–506 (2000). https://doi.org/10.1038/35019537
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35019537
This article is cited by
-
Activin is a neural inducer of a male-specific muscle in Drosophila
Scientific Reports (2024)
-
Evolution of a neuromuscular sexual dimorphism in the Drosophila montium species group
Scientific Reports (2021)
-
The transformer-2 and fruitless characterisation with developmental expression profiles of sex-determining genes in Bactrocera dorsalis and B. correcta
Scientific Reports (2020)
-
teiresias, a Fruitless target gene encoding an immunoglobulin-superfamily transmembrane protein, is required for neuronal feminization in Drosophila
Communications Biology (2020)
-
Partial proteasomal degradation of Lola triggers the male-to-female switch of a dimorphic courtship circuit
Nature Communications (2019)