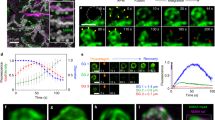
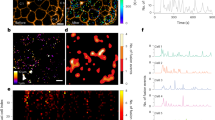
Abstract
Here we report exocytosis of zymogen granules, as examined by multiphoton excitation imaging in intact pancreatic acini. Cholecystokinin induces Ca2+ oscillations that trigger exocytosis when the cytosolic Ca2+ concentration exceeds 1 μM. Zymogen granules fused with the plasma membrane maintain their Ω-shaped profile for an average of 220 s and serve as targets for sequential fusion of granules that are located within deeper layers of the cell. This secondary exocytosis occurrs as rapidly as the primary exocytosis and accounts for most exocytotic events. Granule–granule fusion does not seem to precede primary exocytosis, indicating that secondary fusion events may require a plasma-membrane factor. This sequential-replenishment mechanism of exocytosis allows the cell to take advantage of a large supply of fusion-ready granules without needing to transport them to the plasma membrane.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rothman, J. E. Mechanisms of intracellular protein transport. Nature 372, 55–63 (1994).
Palade, G. Intracellular aspects of the process of protein synthesis. Science 189, 347–358 ( 1975).
Petersen, O. H., Petersen, C. C. H. & Kasai, H. Calcium and hormone action. Annu. Rev. Physiol. 56, 297–319 ( 1994).
Hansen, N. J., Antonin, W. & Edwardson, J. M. Identification of SNAREs involved in regulated exocytosis in the pancreatic acinar cell. J. Biol. Chem. 274, 22871–22876 (1999).
Albright, T. D., Jessell, T. M., Kandel, E. R. & Posner, M. I. Neural science: a century of progress and the mysteries that remain. Neuron 25, S1–S55 ( 2000).
Kasai, H. & Takahashi, N. Multiple kinetic components and the Ca2+ requirements of exocytosis. Phil. Trans. R. Soc. Lond. B 354, 331–335 (1999).
Ichikawa, A. Fine structural changes in response to hormonal stimulation of the perfused caine pancreas. J. Cell Biol. 24, 369– 385 (1965).
Amsterdam, A., Ohad, I. & Schubart, U. K. Dynamic changes in the ultrastrucure of the acinar cell of the rat parotid gland during the secretory cycle. J. Biochem. 41, 753–773 ( 1969).
Padfield, P. J. & Panesar, N. Ca2+ dependent amylase secretion from SLO-permeabilized rat pancreatic acini requires diffusible cytosolic proteins. Am. J. Physiol. 269, G647–G652 (1995).
Ito, K., Miyashita, Y. & Kasai, H. Micromolar and submicromolar Ca2+ spikes regulating distinct cellular functions in pancreatic acinar cells. EMBO J. 16, 242–251 ( 1997).
Segawa, A. Measurement of secretion in confocal microscopy. Methods Enzymol. 307, 328–340 ( 1999).
Ito, K., Miyashita, Y. & Kasai, H. Kinetic control of multiple forms of Ca2+ spikes by inositol trisphosphate in pancreatic acinar cells. J. Cell Biol. 146, 405–414 (1999).
Thorn, P., Lawrie, A. M., Smith, P. M., Gallacher, D. V. & Petersen, O. H. Local and global Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate . Cell 74, 661–668 (1993).
Padfield, P. J. & Panesar, N. Cholecystokinin octapeptide inhibits Ca2+-dependent amylase secretion from permeabilized pancreatic acini by blocking the MgATP-dependent priming of exocytosis. Biochem. J. 330, 329– 334 (1998).
Williams, R. M., Shear, J. B., Zipfel, W. R., Maiti, S. & Webb, W. W. Mucosal mast cell secretion processes imaged using three-photon microscopy of 5-hydroxytryptamine autofluorescence . Biophys. J. 76, 1835– 1846 (1999).
Vogel, S. & Zimmerberg, J. Proteins on exocytotic vesicles mediate calcium-triggered fusion. Proc. Natl Acad. Sci. USA 89, 4749–4753 (1992).
Segawa, A. & Riva, A. Dynamics of salivary secretion studied by confocal laser and scanning electron microscopy. Eur. J. Morphol. 34, 215–219 ( 1996).
Padfield, P. J. & Panesar, N. MgATP acts before Ca2+ to prime amylase secretion from permeabilized rat pancreatic acini. Am. J. Physiol. 273, G655– G660 (1997).
Chestkov, V. V., Radko, S. P., Cho, M. S., Chrambach, A. & Vogel, S. S. Reconstitution of calcium-triggered membrane fusion using `reserve' granules. J. Biol. Chem. 273, 2445–2451 (1998).
Xu, Z., Sato, K. & Wickner, W. LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion . Cell 93, 1125–1134 (1998).
Kasai, H. et al. Multiple and diverse forms of regulated exocytosis in wild-type and defective PC12 cells. Proc. Natl Acad. Sci. USA 96, 945–949 (1999).
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy . Science 248, 73–76 (1990).
Tse, A., Tse, F. W., Almers, W. & Hille, B. Rhythmic exocytosis stimulated by GnRH-induced calcium oscillations in rat gonadotropes. Science 260, 82–84 ( 1993).
Maruyama, Y. & Petersen, O. H. Delay in granular fusion evoked by repetitive cytosolic Ca2+ spikes in mouse pancreatic acinar cells. Cell Calcium 16, 419– 430 (1994).
Anderson, P., Slorach, S. A. & Uvnas, B. Sequential exocytosis of storage granules during antigen-induced histamine release from sensitized rat mast cells in vitro. An electron microscopic study. Acta Physiol. Scand. 88, 359–372 (1973).
Forte, J. G., Black, J. A., Forte, T. M., Machen, T. E. & Wolosin, J. M. Ultrastructural changes related to functional activity in gastric oxyntic cells. Am. J. Physiol. 241, G349-G358 (1981).
Alvarez, D. T. & Fernandez, J. M. Compound versus multigranular exocytosis in peritoneal mast cells. J. Gen. Physiol. 95, 397–409 ( 1990).
Dvorak, A. M. et al. Anaphylactic degranulation of guinea pig basophilic leukocytes. I. Fusion of granule membranes and cytoplasmic vesicles formation and resolution of degranulation sacs. Lab. Invest. 44, 174–191 (1981).
Tai, P. C. & Spry, C. J. The mechanisms which produce vacuolated and degranulated eosinophils. Br. J. Haematol. 49, 219–226 (1981).
Scepek, S. & Lindau, M. Focal exocytosis by eosinophils — comound exocytosis and cumulative fusion. EMBO J. 12 , 1811–1817 (1993).
Angleson, J. K., Cochilla, A. J., Kilic, G., Nussinovitch, I. & Betz, W. J. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nature Neurosci. 2, 440–446 (1999).
Kasai, H. Comparative biology of exocytosis:implications of kinetic diversity for secretory function. Trends Neurosci. 22, 88– 93 (1999).
Wolf, D. E. Designing, building, and using a fluorescence recovery after photobleaching instrument. Methods Cell Biol. 30, 271– 306 (1989).
Drenckhahn, D. & Mannherz, H. G. Distribution of actin and the actin-associated proteins myosin, tropomyosin, alpha-actinin, vinculin, and villin in rat and bovine exocrne glands. Eur. J. Cell Biol. 30, 167–176 ( 1983).
Fox, G. Q. A morphometric analysis of exocytosis in KCl-stimulated bovine chromaffin cells. Cell Tissue Res. 284, 303– 316 (1996).
Orci, L. & Malaisse, W. Hypothesis: single and chain release of insulin secretory granules is related to anionic transport at exocytotic sites. Diabetes 29, 943– 944 (1980).
Furuya, S., Edwards, C. & Ornberg, R. L. Exocytosis of bovine chromaffin granules in Ficoll captured by rapid freezing. J. Electron Microsc. 38 , 143–147 (1989).
Baker, P. & Knight, D. E. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Phil. Trans. R. Soc. Lond. B 296, 83–103 (1981).
Rosenboom, H. & Lindau, M. Exo–endocytosis and closing of the fission pore during endocytosis in single pituitary nerve terminals internally perfused with high calcium concentrations. Proc. Natl Acad. Sci. USA 91, 5267–5271 (1994).
Thomas, P., Lee, A. K., Wong, J. G. & Almers, W. A triggered mechanism retrieves membrane in seconds after Ca2+-stimulated exocytosis in single pituitary cells. J. Cell Biol. 124, 667–675 (1994).
Heuser, J. E. & Reese, T. S. Structural changes after transmitter release at the frog neuromuscular junction. J. Cell Biol. 88, 564–580 (1981).
Acknowledgements
This work was supported by Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Corporation (JST), and by the Research for the Future program of the Japan Society for the Promotion of Science (JSPS), of which K.I. is a research fellow.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Figure S1
Comparison of depth penetration between two-photon excitation imaging and confocal imaging. a, b, Images of an acinar preparation stained with Oregon green 488 BAPTA-1-AM, acquired by x–z scanning with two-photon laser-scanning microscopy (a) or confocal laser-scanning microscopy (b). Luminal structures are apparent in the two-photon excitation image, but not in the confocal image. Confocal imaging (Fig. 1b, d) was carried out using the same scanning microscope and objective lens, but an argon laser was used for excitation at 488 nm and fluorescence of Oregon green 488 BAPTA-1 was measured at 500–600 nm through a pinhole (Olympus, CA2). c, Stereo-pair of two-photon images, obtained by xndash;yndash;z scanning of an acinar preparation that had been immersed in 0.5 mM SRB for 20 min and stimulated with 10 pM CCK for 5 min. Many W-shaped profiles of fused zymogen granules are visible adjacent to lumens, but not to basolateral membranes. (PDF 285 kb)
Rights and permissions
About this article
Cite this article
Nemoto, T., Kimura, R., Ito, K. et al. Sequential-replenishment mechanism of exocytosis in pancreatic acini . Nat Cell Biol 3, 253–258 (2001). https://doi.org/10.1038/35060042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35060042
This article is cited by
-
Membrane transformations of fusion and budding
Nature Communications (2024)
-
Loss of the ciliary protein Chibby1 in mice leads to exocrine pancreatic degeneration and pancreatitis
Scientific Reports (2021)
-
Granuphilin exclusively mediates functional granule docking to the plasma membrane
Scientific Reports (2016)
-
Post-fusion structural changes and their roles in exocytosis and endocytosis of dense-core vesicles
Nature Communications (2014)
-
Multiple roles for the actin cytoskeleton during regulated exocytosis
Cellular and Molecular Life Sciences (2013)