Abstract
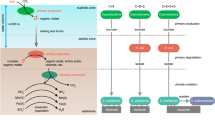
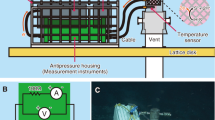
In many marine environments, a voltage gradient exists across the water–sediment interface resulting from sedimentary microbial activity. Here we show that a fuel cell consisting of an anode embedded in marine sediment and a cathode in overlying seawater can use this voltage gradient to generate electrical power in situ. Fuel cells of this design generated sustained power in a boat basin carved into a salt marsh near Tuckerton, New Jersey, and in the Yaquina Bay Estuary near Newport, Oregon. Retrieval and analysis of the Tuckerton fuel cell indicates that power generation results from at least two anode reactions: oxidation of sediment sulfide (a by-product of microbial oxidation of sedimentary organic carbon) and oxidation of sedimentary organic carbon catalyzed by microorganisms colonizing the anode. These results demonstrate in real marine environments a new form of power generation that uses an immense, renewable energy reservoir (sedimentary organic carbon) and has near-immediate application.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yen, T.F. Chemical aspects of marine sediments. in Chemistry of Marine Sediments (ed. Yen, T.F.) 1–38 (Ann Arbor Science Publishers, Ann Arbor, MI, 1977).
Cai, W.-J. & Reimers, C.E. Benthic oxygen flux, bottom water oxygen concentration and core top organic carbon content in the deep northeast Pacific Ocean. Deep-Sea Res. I 42, 1681–1699 (1995).
Froelich, P.N. et al. Early oxidation of organic-matter in pelagic sediments of the eastern equatorial Atlantic–suboxic diagenesis. Geochim. Cosmochim. Acta 43, 1075–1090 (1979).
Berner R.A. Early Diagenesis: A Theoretical Approach (Princeton Univ. Press, Princeton, NJ, 1980), p. 241.
Morel, F.M.M. & Hering, J.G. Principles and Applications of Aquatic Chemistry (Wiley, New York, 1993), p. 588.
Schindler, J.E. & Honick, K.R. Oxidation-reduction determinations at mud-water interface. Limnol. Oceanogr. 16, 837–840 (1971).
Reimers, C.E., Tender, L.M., Fertig, S.J. & Wang, W. Harvesting energy from the marine sediment-water interface. Environ. Sci. & Technol. 35, 192–195 (2001).
Palmore, G.T.T. & Whitesides, G.M. Enzymatic conversion of biomass for fuels production. ACS Sym. Ser. 566, 271–290 (1994).
Wilcock, W.S.D. & Kauffman, P.C. Development of a seawater battery for deep-water applications. J. Power Sources 66, 71–75 (1997).
Hart, A.B. & Womack, J.G. Fuel Cells. Theory and Application (Chapman and Hall, London, 1967), p. 54.
Bard, A.J. & Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications (Wiley, New York, 1980), p. 130.
Boudreau, B.P. Diagenetic Models and their Implementation. Modelling Transport and Reactions in Aquatic Sediments (Springer, New York, 1997), p. 414.
Zhang, L.Z., Lever, A.B.P. & Pietro, W.J. Surface copper immobilization by chelation of alizarin complexone and electrodeposition on graphite electrodes, and related hydrogen-sulfide electrochemistry; matrix-isolation of atomic copper and molecular copper sulfides on a graphite electrode. J. Electroanal. Chem. 385, 191–200 (1995).
Bond, D.R., Holmes, D.E., Tender, L.M. & Lovley, D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295, 483–485 (2002).
Lovley, D.R et al. Humic substances as electron acceptors for microbial respiration. Nature 382, 445–448 (1996).
Lovley, D.R. & Phillips, E.J.P. Novel processes for anaerobic sulfate production from elemental sulfur by sulfate-reducing bacteria. Appl. Environ. Microbiol. 60, 2394–2399 (1994).
Finster, K., Liesack, W. & Thamdrup, B. Elemental sulfur and thiosulfate disproportionation by Desulfocapsa sulfoexigens sp. nov., a new anaerobic bacterium isolated from marine surface sediment. Appl. Environ. Microbiol. 64, 119–125 (1998).
Sahling, H., Rickert, D., Linke, P., Suess, E. & Lee, R.W. Macrofaunal community structure and sulfide flux at gas hydrate deposits from the Cascadia convergent margin, NE Pacific. Mar. Ecol. Prog. Ser. 231, 121–138 (2002).
Kennett, J.P., Cannariato, K.G., Hendy, I.L. & Behl, R.J. Carbon isotopic evidence for methane hydrate instability during quaternary interstadials. Science 288, 128–133 (2000).
Ausubel, F.M. et al. Protocols in Molecular Biology (ed. Chanda, V.B.) (Wiley, New York, 1997).
Lane, D.L. in Nucleic Acid Techniques in Bacterial Systematics (eds Stackebrandt, E. & Goodfellow, M.) 115–175 (Wiley, Chichester, United Kingdom, 1991).
Marchesi, J.R. et al. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 64, 795–799 (1998).
Lane, D.L. et al. Rapid-determination of 16S ribosomal-RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 82, 6955–6959 (1985).
Altschul, S.F. et al. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Maidak, B.L. et al. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27, 171–173 (1999).
Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14, 454–458 (1969).
Measures, C.I., Yuan, J. & Resing, J.A. Determination of iron in seawater by flow injection-analysis using in-line preconcentration and spectrophotometric detection. Mar. Chem. 50, 3–12 (1995).
Acknowledgements
This work was supported by grants from the Office of Naval Research (ONR), the Naval Research Laboratory (NRL), and the Defense Advanced Research Projects Administration (DARPA). We are grateful to Rose Petrecca, Joe Debarro, and staff of the Tuckerton, New Jersey Field Station of Rutgers University, Institute of Marine and Coastal Sciences for assistance in fuel-cell deployment and retrieval. We also thank M. Sommer, R. Emmett, T. Bridgeman, T. Builder, W. Hanshumacher, D. Jacobson, and M. Spencer for diving assistance at the Newport site and L. Annable for field assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tender, L., Reimers, C., Stecher, H. et al. Harnessing microbially generated power on the seafloor. Nat Biotechnol 20, 821–825 (2002). https://doi.org/10.1038/nbt716
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt716
This article is cited by
-
Affordable ESP32-based monitoring system for microbial fuel cells: real-time analysis and performance evaluation (ESP32-based data logger as a monitoring system for microbial fuel cell)
International Journal of Energy and Water Resources (2023)
-
Electricigens and microbial fuel cells for bioremediation and bioenergy production: a review
Environmental Chemistry Letters (2021)
-
Sediment microbial fuel cells as a barrier to sulfide accumulation and their potential for sediment remediation beneath aquaculture pens
Scientific Reports (2020)