Abstract
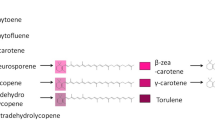
We have used combinatorial biosynthesis to synthesize novel lipophilic carotenoids that are powerful cellular antioxidants. By co-expressing three different carotenoid desaturases in combination with a carotenoid hydratase, a cyclase, and a hydroxylase on compatible plasmids in Escherichia coli, we synthesized four novel carotenoids not previously detected in biological material or chemically synthesized. Their identification was based on their relative retention times on HPLC, spectroscopic properties, molecular weights, number of hydroxy groups, and 1H-NMR spectra. The carotenoids were designated as 1-HO-3′, 4′-didehydrolycopene, 3, 1′-(HO)2-γ-carotene, 1,1′-(HO)2-3, 4, 3′, 4′-tetradehydrolycopene, and 1, 1′-(HO)2-3, 4-didehydrolycopene. These novel acyclic derivatives differ from structurally related compounds by extension of the conjugated polyene chain as well as additional hydroxy groups at position C-1′. We determined their antioxidative activity in a liposome-membrane model system, which showed that their ability to protect against photooxidation and radical-mediated peroxidation reactions was linked to the length of the conjugated double-bond system and the presence of a single hydroxy group. The protection of membrane degradation was superior to the related 1-HO and 1, 1′-(HO)2 lycopene derivatives, making them interesting pharmaceutical candidates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Johnson, E.A. & Schroeder, W. Microbial carotenoids. A. Adv. Biochem. Eng. Biotechnol. 53, 119–178 (1995).
Krinsky, N.I. The biological properties of carotenoids. Pure Appl. Chem. 66, 1003–1010 (1994).
Conn, P.F. et al. Carotene–oxygen radical interactions. Free Rad. Res. Comms. 16, 401–408 (1992).
Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 9, 1551–1558 (1995).
Woodall, A.A., Britton, G. & Jackson, M.J. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: relationship between carotenoid structure and protective ability. Biochim. Biophys. Acta 1336, 575–586 (1997).
Hirayama, O. et al. Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids 29, 149–150 (1994).
Ourisson, G. & Nakatani, Y. Bacterial carotenoids as membrane reinforcers. In Carotenoids: chemistry and biology (eds Krinsky, N.I., Mathews-Roth, M.M. & Taylor, R.F.) 237–245 (Plenum Press, New York, NY; 1989).
Sandmann, G. et al. The biotechnological potential and design of novel carotenoids by gene combination in Escherichia coli. Trends Biotechol. 17, 233–237 (1999).
Albrecht, M. et al. Synthesis of atypical cyclic and acyclic hydroxy carotenoids in Escherichia coli transformants. J. Biotechnol. 58, 177–185 (1997).
Takaichi, S. Usefulness of field desorption mass spectrometry in determining molecular masses of carotenoids, natural carotenoid derivatives and their chemical derivatives. Org. Mass Spectrom. 28, 785–788 (1993).
Englert, G. NMR spectroscopy. In Carotenoids, Vol. 1B (eds Britton, G., Liaaen-Jensen, S. & Pfander, H.) 147–260 (Birkhäuser Verlag, Basel; 1995).
Hahn, F.M., Baker, J.A. & Poulter, C.D. Open reading frame 176 in the photosynthesis gene cluster of Rhodobacter capsulatus encodes idi, a gene for isopentenyl diphosphate isomerase. J. Bacteriol. 178, 619–624 (1996).
Sprenger, G.A. et al. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-D-xylulose 5-phosphate precursor to isoprenoids, thiamin and pyridoxal. Proc. Natl. Acad. Sci. USA 94, 12857–12862 (1997).
Canfield, L.M., Forage, J.W. & Valenzuela, J.G. Carotenoids as cellular antioxidants. Proc. Soc. Exp. Biol. Med. 200, 260–265 (1992).
Gerster, H. Anticarcinogenic effect of common carotenoids. Int. J. Vit. Nutr. Res. 63, 93–121 (1992).
Rice-Evans, C.A. Why do we expect carotenoids to be antioxidants in vivo? Free Rad. Res. 26, 381–398 (1997).
Miller, N.J. et al. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 384, 240–242 (1996).
Subczynski, W.K., Markowska, E. & Sielewiesiuk, J. Effects of polar carotenoids on the oxygen diffusion–concentration product. An EPR spin label study. Biochim. Biophys. Acta 1068, 68–72 (1991).
Kovach et al. Four new derivatives of the broad-host range cloning vector pBBR1MCS, carrying different antibiotic resistance cassettes. Gene 166, 175–176 (1995).
Schmidhauser, T.J., Lauter, F.R., Russo, V.E.A. & Yanofsky, C. Cloning, sequence, and photoregulation of al-1, a carotenoid biosynthetic gene of Neurospora crassa. Mol. Cell. Biol. 10, 5064–5070 (1990).
Linden, H., Vioque, A. & Sandmann, G. Isolation of a carotenoid biosynthesis gene coding for ζ-carotene desaturase from Anabaena PCC7120. FEMS Microbiol. Lett. 106, 99–104 (1993).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular cloning: a laboratory manual. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY; 1989).
Ernst, S. & Sandmann, G. Poly-cis carotene pathway in the Scenedesmus mutant C-6D. Arch. Microbiol. 150, 590–594. (1988).
Davis, B.H. Carotenoids. In Biochemistry of plant pigments, Vol. 2 (ed. Goodwin, T.W.) 38–365 (Academic Press, London; 1976).
Takaichi, S. et al. The carotenoid 7,8-dihydro- end group can be cyclized by the lycopene cyclases from the bacterium Erwinia uredovora and the higher plant Capsicum annuum. Eur. J. Biochem. 241, 291–296 (1996).
Takaichi, S. & Shimada, K. Characterization of carotenoids in photosynthetic bacteria. Methods Enzymol. 213, 374–385 (1992).
Steiger, S., Schäfer, L. & Sandmann, G. High-light upregulation of carotenoids and their antioxidative properties in the cyanobacterium Synechocystis PCC 6803. J. Photochem. Photobiol. B 521, 14–18 (1999).
Buege, J.A. & Aust, S.D. Microsomal lipid peroxidation, Methods Enzymol., 52, 302–310 (1978).
Misawa, N. et al. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J. Bacteriol. 177, 6575–6584 (1995).
Albrecht, M., Ruther, A. & Sandmann, G. Purification and biochemical characterization of a hydroxyneursporene desaturase involved in the biosynthetic pathway of the carotenoid spheroidene in Rhodobacter spheroides. J. Bacteriol. 179, 7462–7467 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albrecht, M., Takaichi, S., Steiger, S. et al. Novel hydroxycarotenoids with improved antioxidative properties produced by gene combination in Escherichia coli. Nat Biotechnol 18, 843–846 (2000). https://doi.org/10.1038/78443
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/78443
This article is cited by
-
Genetically engineered biosynthetic pathways for nonnatural C60 carotenoids using C5-elongases and C50-cyclases in Escherichia coli
Scientific Reports (2019)
-
Biotransformation of Waste Glycerol from Biodiesel Industry in Carotenoids Compounds by Halophilic Microorganisms
Waste and Biomass Valorization (2019)
-
Trennung schwer löslicher Carotinoide mit umweltschonenden Lösungsmitteln
BIOspektrum (2019)
-
Analysis of Novel Antioxidant Sesquarterpenes (C35 Terpenes) Produced in Recombinant Corynebacterium glutamicum
Applied Biochemistry and Biotechnology (2018)
-
On the current role of hydratases in biocatalysis
Applied Microbiology and Biotechnology (2018)