Abstract
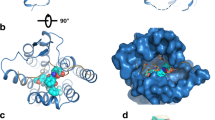
Pairs of cystine residues were introduced in the α- and β-subunits of human choriogonadotropin at positions with optimal geometries for the formation of disulfide bonds. Using the homology with luteiniz-ing hormone and follicle stimulating hormone, similar mutations were carried out in these glycoprotein hormones. In nearly all mutants the corresponding disulfide bonds were formed leading to a non-natural, covalent linkage between the α- and β-subunits. The mutants typically display wild-type receptor binding and bioactivity. The mutants with non-natural intersubunit disulfide bonds display enhanced thermostabilities relative to the corresponding heterodimeric glycoprotein hormones, rendering them candidates for long acting gonadotropins with enhanced shelf lives.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Combarnous, Y. 1992. Molecular basis of the specificity of binding of glycoprotein hormones to their receptors. Endocr Rev. 13: 670–691.
Lapthorn, A. J., Harri, D. C., Littlejohn, A. Lustbader, J. W., Canfield, R. E., Machin K. J., et al. 1994. Crystal structure of human chononic gonadotropin. Nature 369: 455–461.
Wu, H., Lustbader, J.W., Liu Y., Canfield, R. E., and Hendrickson W.A. 1994. Structure of the human chorionic gonadotropin at 2.6 Å resolution from MAD analysis of the selenomethionyl protein. Structure 2: 545–558.
Isaacs, N. W. 1995. Cystine knots. Curr. Opin. Struct. Biol. 5: 391–395.
Pierce, J. and Parsons, T. 1981. Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 50: 465–495.
Sugahara, T., Pixley, M. R., Minami, S., Perlas, E., Ben-Menahem, D., Hsueh, A. J. W., and Boime, I. 1995. Biosynthesis of a biologically active single chain containing the human common α and chononic gonadotropm β subunits in tandem. Proc Nail Acad Set USA 92: 2041–2045.
Narayan, P., Wu, C., and Puett, D. 1995. Functional expression of yoked human chorionic gonadotropin in baculovirus-infected insect cells. Mol. Endocrinol. 9: 1720–1726.
Ben-Menahem, D. and Boime, I. 1996. Converting heterodimeric gonadotropins to genetically linked single chains. Trends Endocnnol. Metab. 7: 100–105.
Sugahara, T., Sato, A., Kudo, M., Ben-Menahem, D., Pixley, M. R., Hsueh, A. J. W., and Boime, I. 1996. Expression of biologically active fusion genes encoding the common α subunit and the follicle-stimulating β subunit. J. Biol. Chem. 271: 10445–10448.
Sugahara, T., Grootenhuis, P.D.J., Sato, A., Kudo, M., Ben-Menahem, D., Pixley, M. R., et al. 1996. Expression of biologically active fusion genes encoding the common α subunit and either the CG β or FSH β subunits role of a linker sequence. Mol. Cell Endocrinol. 125: 71–77.
Heikoop, J., van Beuningen-de Vaan, M. J. A. C. M., van den Boogaart, P., and Grootenhuis, P.D.J. 1997. Single chain human gonadotropins evaluation of subunit truncation and the nature of the spacer. Eur. J. Biochem. 245: 656–662.
Shaw, A. and Bott, R. 1996. Engineering enzymes for stability. Curr. Opm. Struct. Biol. 6: 546–550.
Matsumara, M. and Matthews, B.W. 1991. Stabilization of functional proteins by introduction of multiple disulfide bonds. Meth. Enzymol. 202: 336–356.
Creighton, T.E., Zapun, A., and Darby, N. J. 1995. Mechanisms and catalysts of disulphide bond formation in proteins. Trends Biotechnol. 13: 18–23.
Creighton, T.E., Darby, N. J., and Kemmink, J. 1996. The roles of partly folded intermediates in protein folding. FASEB J. 10: 110–118.
Ruddon, R.W., Sherman, S.A., and Bedows, E. 1996. Protein folding in the endoplasmatic reticulum: lessons from the human chorionic gonadotropin β subunit. Protein Sci. 5: 1443–1452.
Blundell, T.L. 1994. Problems and solutions in protein engineering—towards rational design. Trends Biotechnol. 12: 145–148.
Hazes, B. and Dijkstra, B. W. 1988. Model building of disulfide bonds in proteins with known three-dimensional structure. Protein Eng. 2: 119–125.
Boime, I., Ben-Menahem, D., Kudo, M., Sugahara, T., Sato, A., and Hsueh, A. J. W. 1997. Conversion of heterodimeric gonadotropins to genetically linked single chains new approaches to structure-activity relationships and analog design, in FSH Action and Intraovarian Regulation Parthenon Publishing Casterton Hall, UK.
Matzuk, M. M., Keene, J. L., and Boime, I. 1989. Site specificity of the chononic gonadotropin N-linked oligosaccharides in signal transduction. J. Biol. Chem. 264: 2409–2414.
Valove, F.M., Finch, C., Anasti, J. N., Froehlich, J., and Flack, M.R. 1994. Receptor binding and signal transduction are dissociable functions requiring different sites on follicle stimulating hormone. Endocrinol. 135: 2657–2661.
Puett, D., Huang, J., and Xia, H. 1994. Delineation of subunit and receptor contact sites by site-directed mutagenesis of hCG β, pp 122–134 in Glycoprotein Hormones. Lustbader, J. W., Puett, D. and Ruddon, R. W. (eds) Sprmger-Verlag, New York
Chen, F. and Puett, D. 1992. A single amino acid residue replacement in the β subunit of human chononic gonadotrophm results in the loss of biological activity. J. Mol. Endocrinol. 8: 87–89.
Pace, C.N. 1990. Conformational stability of globular proteins. Trends Biotechnol. 15: 14–17.
Higuchi, R. 1989 Using PCR to engineer DNA, pp. 61–70 in PCR Technology, Principles and Applications for DNA Amplification. Erlich, H. A. (ed.) Stockton Press, New-York.
Olijve, W., De Boer, W., Mulders, J.W.M., and Van Wezenbeek P.M.G.F. 1996. Molecular biology and biochemistry of human recombinant follicle stimulating hormone (Puregon). Mol. Hum. Reprod. 2: 371–382.
Talmadge, K., Vamvakopoulos N. C., and Fiddes, J. C. 1984. Evolution of the genes for the β subunits of human chononic gonadotropm and luteinizing hormone. Nature 307: 37–40.
Fiddes, J. C. and Goodman H. M. 1980. The cDNA for the β-subunit of human chorionic gonadotropin suggests evolution of a gene by readthrough into the 3′-untranslated region. Nature 286: 684–687.
Jia, X. -C., Oikawa, M., Bo, M., Tanaka, T., Ny, T., Boime, I, and Hsueh A. J. W. 1991. Expression of human luteinizing hormone (LH) receptor: interaction with LH and chononic gonadotropm from human but not equine, rat, and ovine species. Mol. Endocrinol. 5: 759–768.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heikoop, J., Boogaart, P., Mulders, J. et al. Structure-based design and protein engineering of intersubunit disulfide bonds in gonadotropins. Nat Biotechnol 15, 658–662 (1997). https://doi.org/10.1038/nbt0797-658
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0797-658