Abstract
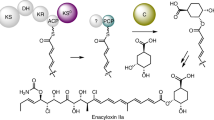
By the use of a semi-purified preparation of isopenicillin N synthetase (“cyclase”) from Cephalosporium acremonium, a new penicillin was prepared enzymatically. The conversion of a sulfur analog of the normal tripeptide precursor (LLD-ACV) to the sulfur analog of isopenicillin N takes place in minutes. Such enzymatic reactions will be economically used in the future to generate new β-lactam antibiotics, now made by expensive synthetic chemistry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Demain, A.L. 1983. Biosynthesis of β-lactam antibiotics. p. 189–228. In: Antibiotics Containing the β-lactam Structure. A.L. Demain and N.A. Solomon (eds.), Springer-Verlag, Heidelberg.
Hollander, I.J., Shen, Y.-Q., Heim, J., Wolfe, S., and Demain, A.L. 1984. A pure enzyme catalyzing penicillin biosynthesis. Science 224: 610–612.
Kupka, J., Shen, Y.-Q., Wolfe, S., and Demain, A.L. 1983. Studies on the ring-cyclization and ring-expansion enzymes of β-lactam biosynthesis in Cephalosporium acremonium . Can. J. Microbiol. 29: 488–496.
Kupka, J., Shen, Y.-Q., Wolfe, S., and Demain, A.L. 1983. Partial purification and properties of the α-ketoglutarate-linked ring-expansion enzyme of β-lactam biosynthesis of Cephalosporium acremonium . FEMS Microbiol. Lett. 16: 1–6.
Troonen, H., Roelants, P., and Boon, B. 1976. RIT 2214, a new biosynthetic penicillin produced by a mutant of Cephalosporium acremonium . J. Antibiot. 29: 1258–1267.
Iwamatsu, K., Inouye, S., Tsuruoka, T., Mizutani, K., Omoto, S., Ogino, H., Miyauchi, K., Watanabe, T., and Niida, T. 1983. Synthesis and biological activity of 7β-(2-amino-2-carboxy)-ethylthioacetamido-7-αpha;-methoxy cephalosporin derivatives. J. Antibiot. 36: 229–241.
Michaelis, L. and Schubert, M.P. 1934. The reaction of iodoacetic acid on mercaptans and amines. J. Biol. Chem. 160: 331–341.
Wolfe, S. Studies related to beta-lactam compounds, p. 101–114. In: Current Trends in Organic Synthesis. H. Nozaki (ed.), Pergamon Press, Oxford.
Wolfe, S. and Jokinen, M.G. 1979. Total synthesis of δ-(L-α-amino-adipyl)-L-cysteinyl-D-valine (ACV), a biosynthetic precursor of penicillin and cephalosporin. Can. J. Chem. 57: 1388–1399.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wolfe, S., Hollander, I. & Demain, A. Enzymatic Synthesis of a Sulfur-Analog of Penicillin Using the “cyclase” of Cephalosporium Acremonium. Nat Biotechnol 2, 635–636 (1984). https://doi.org/10.1038/nbt0784-635
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0784-635