Abstract
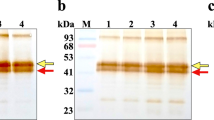
A systematic screen of human kidney tissue samples has resulted in the establishment of a permanent cell line, TCL-598, which produces and secretes into the medium milligrams per liter quantities of a urokinase-like plasminogen activator. Analysis of the properties of this enzyme indicate that it is a single polypeptide chain of approximately 50,000 molecular weight, it exhibits strong affinity for fibrin, and it is inactive. In the presence of plasmin, however, it is converted into an active two chain enzyme indistinguishable from the familiar high molecular weight form of urokinase. We have termed this pro-urokinase derived from TCL-598 kidney plasminogen activator (KPA). The establishment of fibrin/Celite affinity for this inactive precursor suggests a unique potential for KPA in the treatment of acute vascular diseases such as myocardial infarct, stroke, or deep vein thrombosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Astrup, T. 1978. Fibrinolysis: an overview, p. 1–57. In: Progress in Chemical Fibrinolysis and Thrornbolysis, Vol. 3, Davidson, Rowan, Samana and Desnoyers (eds.), Raven Press, N.Y.
Rijken, D.C., Hoylaerts, M., and Collen, D. 1982. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J. Biol. Chem. 257: 2920–2925.
Wun, T.-C., Ossowski, L., and Reich, E. 1982. A pro-enzyme form of human urokinase. J. Biol. Chem. 257: 7262–7268.
Nielsen, L.S., Hansen, J.G., Skriver, L., Wilson, E.L., Kaltoft, K., Zeuthen, J., and Dano, K. 1982. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry 21: 6410–6415.
Husain, S.S., Gurewich, V., and Lipinski, B. 1983. Purification and partial characterization of a single-chain high-molecular-weight form of urokinase from human urine. Arch. Biochem. Biophys. 220: 31–38.
Sumi, H., Maruyama, M., Matsuo, O., Mihara, H., and Toki, N. 1982. High fibrin-binding and thrombolytic properties of single polypeptide chain-high molecular weight urokinase. Thromb. Haemostas. 47: 297–301.
Towbin, H., Staehelin, T., and Gordon, J. 1979. Eiectrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76: 4350–4354.
Gunzler, W., Steffens, G., Otting, F., Buse, G., and Flohe, L. 1982. Structural relationship between human high and low molecular weight mass urokinase. Hoppe-Seyler's Z. Physiol. Chem. 363: 133–141.
Gunzler, W., Steffens, G., Otting, F., Kim, S.-M., Frankus, E., and Flohe, L. 1982. The primary structure of high molecular mass urokinase from human urine. The complete amino acid sequence of the A chain. Hoppc-Seyler's Z. Physiol. Chem. 363: 1155–1165.
Henschcn, A., Reich, E., Sauser, D., and Lottspeich, F. 1981. p. 367–370. In: Verhandlungsber, d. 25. Tag. d. Dlsch. Arbeitsgem. f. Blutgefinnungsforsch. in Munchcn, Blumel, G. and Haas, S. (eds.), Schattauer-Verlag, Stuttgart.
Ong, E.B., Soberano, M.E., Johnson, A.J., and Schoellmann, G. 1977. Studies on the biochemistry of urokinase. Thromb. Haemostas. 38: 801–808.
Husain, S.S., Lipinski, B., and Gurewich, V. 1981. Rapid purification of a high affinity plasminogen activator from human blood plasma by specific adsorption on fibrin celite. Proc. Nat. Acad. Sci. USA 78: 4265–4269.
Lijnen, H.R. and Collen, D. 1982. Interaction of plasminogen activators and inhibitors with plasminogen and fibrin. Seminars in Thromb. Haemostas. 8: 2–10.
Granelli-Piperno, A. and Reich, E. 1978. A study of proteases and protease-inhibitor complexes in biological fluids. J. Exp. Med. 148: 223–234.
White, W.F., Barlow, G.H., and Mozen, M.M. 1966. The isolation and characterization of plasminogen activators (urokinase) from human urine. Biochemistry 5: 2160–2169.
Gross, E. and Witkop, B. 1961. Selective cleavage of the methionyl peptide bonds in ribomiclease with cyanogen bromide. J. Am. Chem. Soc. 83: 1510–1514.
Edman, P. and Begg, G. 1967. A protein sequenator. Eur. J. Biochem. 1: 80–91.
Sauer, R.T., Pan, J., Hopper, P., Hehir, K., Brown, J., and Poteete, A.R. 1981. Primary structure of the phage P22 rcpressor and its gene c2. Biochemistry 20: 3591–3598.
Weber, K. and Osborn, M. 1969. The reliability of molecular weight determinations by dodecyl sulfate—polyacrylamide gel electrophoresis. J. Biol. Chem. 244: 4406–4412.
Brakman, P. 1967. Fibrinolysis: A sianclardized fibrin plate method and a librinolytii: assay of plasminogen. Scheltema and Holkema, Amsterdam
Gurewich, V., Pannell, R., Louie, S., Kelley, P., Suddith, R.L., and Greenlee, R. 1984. Effective and fibrin-specific clot lysis by a zymogen precursor form of urokinase (pro-urokinase). J. Clin. Invest. 73: 1731–1739.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kohno, T., Hopper, P., Lillquist, J. et al. Kidney Plasminogen Activator: A Precursor Form of Human Urokinase With High Fibrin Affinity. Nat Biotechnol 2, 628–634 (1984). https://doi.org/10.1038/nbt0784-628
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0784-628