Abstract
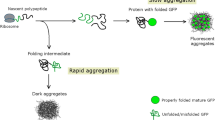
We have studied the cause of inclusion body formation in Escherichia coli grown at 37°C using statistical analysis of the composition of 81 proteins that do and do not form inclusion bodies. Six composition derived parameters were used. In declining order of their correlation with inclusion body formation, the parameters are charge average, turn forming residue fraction, cysteine fraction, proline fraction, hydrophilicity, and total number of residues. The correlation with inclusion body formation is strong for the first two parameters but weak for the last four. This correlation can be used to predict the probability that a protein will form inclusion bodies using only the protein's amino acid composition as the basis for the prediction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Williams, D.C., Van Frank, R.M., Muth, W.L. and Barnett, J.P. 1982. Cytoplasmic inclusion bodies in Escherichia coli producing bio-synthetic human insulin protein. Science 215: 684–687.
Schein, C.H. 1990. Solubility as a function of protein structure and solvent components. Bio/Technology 8: 308–317.
Carrell, R.W., Lehmann, H. and Hutchisdon, H.F. 1966. Haemoglobin Koln (beta 98 valine-methionine): an unstable protein causing inclusion body anemia. Nature 210: 915–916.
Schein, C.H. and Noteborn, M.H.M. 1988. Formation of soluble recombinant proteins in Escherichia coli is favored by lower growth temperature. Bio/Technology 6: 291–294.
Krueger, J.K., Kulke, M.N., Schutt, C. and Stock, J. 1989. Protein inclusion body formation and purification. BioPharm. 2: 40–45.
Marston, F.A.O. 1986. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem. J. 240: 1–12.
Mitraki, A. and King, J. 1989. Protein folding intermediates and inclusion body formation. Bio/Technology 7: 690–697.
Schein, C.H. 1989. Production of soluble proteins in bacteria. Bio/Technology 7: 1141–1149.
Nagai, K., Thogersen, H.C. and Luisi, B.F. 1988. Refolding and crystallographic studies of eukaryotic proteins produced in Escherichia coli. Biochem. Soc. Trans. 16: 108–110.
Brasnet, A.H., Schoemaker, J.M. and Marston, F.A.O. 1985. Examination of calf prochymosin accumulation in Escherichia coli: disulfide linkages are a structural component of prochymosin containing inclusion bodies. EMBO J. 4: 775–780.
Chou, P.Y. and Fasman, G.D. 1978. Empirical predictions of protein conformation. Ann. Rev. Biochem. 47: 251–276.
Evans, P.A., Dobson, C.M., Kautz, R.A., Hatfull, G. and Fox, R.O. 1987. Proline isomerism in staphylococcal nuclease characterized by NMR and site-directed mutagenesis. Nature 329: 266–268.
Tanford, C. 1958. Physical Chemistry of Macromolecules. John Wiley & Sons, New York.
Neidhardt, F.C., Ingraham, J.L., Low, K.B., Magasanik, B. and Schaechter, M. (Eds.). 1987. Escherichia coli and Salmonella typhimurium. Cellular and Molecular Biology, Volume 2, p. 222–243. American Society for Microbiology. Washington, B.C.
Riddick, T. 1968. Control of Colloid Stability Through Zeta Potential. Livingston Publishing Company, Wynnewood, Pennsylvania.
Kauzmann, W. 1959. Structural factors involved in protein stability. Advances in Protein Chemistry. 14: 19–29.
Arakawa, T. and Timasheff, S.N. 1985. Theory of protein solubility. Meth. Enzym. 114: 49–77.
Hopp, T.P. and Woods, K.R. 1981. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. USA 78: 3824–3828.
Fisher, R.A. 1936. The use of multiple measurements in taxonomic problems. Ann. Eugenics 7: 179–188.
Devereux, J., Haeberli, P. and Smithies, O. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acid Res. 12: 387–395.
King, R.S. and Bryant, J. 1982. Applied Statistics Using the Computer. Mayfield Publishing Company, Palo Alto, CA.
Dixon, W.J. 1988. BMDP Statistical Software Manual. University of California Press, Berkeley.
Hamuda, K., Furisawa, H. and Minagawa, T. 1986. Overproduction and purification of the products of bacteriophage T3 genes 18 and 19. Virology 151: 110–118.
Grumont, R., Worachart, S. and Sant, D.V. 1988. Heterologous expression of the bifunctional thymidylate synthase-dihydrofolate reductase from Leishmania major. Biochemistry 27: 3776–3780.
Bond, J.H., Van Kimnenade, M.W., Schumacher, C. and Kastelein, R.A. 1988. Expression, renaturation, and purification of recombinant human Il-4 in E. coli. Biochem. J. 174: 109–114.
Roberts, J.D. and McMacken, R. 1983. The bacteriophage λO replication protein: isolation and characterization of the amplified initiator. Nucleic Acids Res. 11: 7435–7452.
Cabilly, S., Riggs, A.D., Shivelly, J., Holmes, W., Rey, M., Perry, L.J., Heyneker, H.L. and Wetzel, R. 1984. Generation of antibody activity from immunoglobulin polypeptide chains produced in Escherichia coli. Proc. Natl. Acad. Sci. USA 81: 3273–77.
Sharma, S.K., Evans, D.B., Tomich, C., Cornette, J.C. and Ulrich, R.G. 1987. Folding and reactivation of recombinant human prorenin. Biotechnology and Applied Biochemistry 9: 181–193.
Halenbeck, R., Kawasaki, E., Wrin, J. and Koths, K. Renaturation and purification of biologically active recombinant human macrophage colony-stimulating factor expressed in E. coli. Bio/Technology 7: 710–715.
Bergman, C., Dodt, J., Fink, E., Gassen, H.G. and Kohler, S. 1986. Chemical synthesis and expression of a gene coding for hirudin, the thrombin-specific inhibitor from the leech Hirudo medicinalis. Biol. Chem. Hoppe-Seyler. 367: 731–740.
Weis, J.H., Enquist, L.W., Salstrom, J.S. and Watson, R.J. 1983. An immunologically active chimaeric protein containing herpes simplex virus type 1 glycopotein D. Nature 302: 72–75.
Lunn, C.A., Kathju, S., Wallace, B.J., Kushner, S.R. and Pigiet, V. 1984. Amplification and purification of plasmid-encoded thioredoxin from Escherichia coli K12. J. of Biol. Chem. 259: 10469–10474.
Gross, M., Sweet, R.W., Sathe, G., Yokoyama, S., Fasano, O., Gold-farb, M., Wigler, M. and Rosenberg, M. 1985. Purification and characterization of human H-ras proteins expressed in Escherichia coli. Molecular and Cellular Biology 5: 1015–1024.
Lacal, J.C., Santos, E., Notario, V., Barbacid, M., Yamazaki, S., Kung, H., Seamans, C., McAndrew, S. and Growl, R. 1984. Expression of normal and transforming H-ras genes in Escherichia coli and purification of their encoded p21 proteins. Proc. Natl. Acad. Sci. USA 81: 5305–5309.
Derom, C., Gheysen, D. and Fiers, W. 1982. High level synthesis in Escherichia coli of the SV-40 small t antigen under control of the bacteriophage λpL promoter. Gene 17: 45–54.
Bastia, D., Germino, J., Mukherjee, S. and Vanaman, T. 1986. New approaches to the expression and isolation of a regulatory protein. Genetic Eng. 8: 333–351.
Rand, K.N., Singh, M., Thogersen, H.C. and Gait, M.J. 1986. T4 RNA ligase: New structural studies on an unusual but useful enzyme. Proc. Int. Symp. Biomol. Structural Interactions, Supp. J. Biosci. 8: 89–100.
Ibanez, C., Garcia, J.A., Carrascosa, J.L. and Salas, M. 1984. Overproduction and purification of the connector protein of Bacillus subtilus phage ϕ29. Nucleic Acids Res. 12: 23–66
Ayala, J.A., Pla, J., Desviat, L.R. and De Pedro, M.A. 1988. A lacZ-pbpB gene fusion coding for an inducible hybrid protein that recognizes localized sites in the inner membrane of Escherichia coli. J. Bacteriol. 170: 3333–3341.
Reed, R.R. 1981. Transposon-mediated site-specific recombination: a defined in vitro system. Cell 25: 713–719.
Schulz, M., Buell, G., Schmid, E., Movva, R. and Selzer, G. 1987. Increased expression in Escherichia coli of a synthetic gene incoding human somatomedin C after gene duplication and fusion. J. Bacteriol. 169: 5385–5392.
Maina, C.V., Riggs, P.D., Grandea, A.G., Slatko, B.E., Moran, L.S., Tagliamonte, J.A., McReynolds, L.A. and di Guan, C. 1988. An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from maltose-binding protein. Gene 74: 365–373.
Squires, C.H., Childs, J., Eisenberg, S.P., Polverini, P.J. and Sommer, A. 1988. Production and characterization of human basic fibro-blast growth factor from Escherichia coli. J. Biol. Chem. 263: 16297–16302.
Piatek, M., Laird, W., Bjorn, M.J., Wang, A. and Williams, M. 1988. Expression of soluble and fully functional ricin A chain in Escherichia coli is temperature sensitive. J. Biol. Chem. 263: 4837–4843.
Bishai, W.R., Rappuoli, R. and Murphy, J.R. 1987. High-level expression of a proteolytically sensitive diphtheria toxin fragment in Escherichia coli. J. Bacteriol. 169: 5140–5151.
McCamen, M.T. 1989. Fragments of prochymosin produced in Escherichia coli form insoluble inclusion bodies. J. Bact. 171: 1225–1227.
Haase-Pettingell, C.A. and King, J. 1988. Formation of aggregates from a thermolabile in vivo folding intermediate in P22 tailspike maturation. A model for inclusion body formation. J. Biol. Chem. 263: 4977–4983.
Goeddel, D.V., Kleid, D.G., Bolivar, F., Heyneker, H.L., Yansura, D.G., Ross, M.J., Miozzari, G., Crea, R., Hirose, T., Kraszewski, A., Itakura, K. and Riggs, A.D. 1979. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc. Natl. Acad. Sci. U.S.A. 76: 106–110.
Dykes, C.W., Brookless, A.B., Coomber, B.A., Noble, S.A., Humber, D.C. and Hobden, A.N. 1988. Expression of atrial natriuretic factor as a cleavable fusion protein with chloramphenical acetyltransferase in Escherichia coli. Eur. J. Biochem. 174: 398–410.
Weir, M.P. and Sparks, J. 1987. Purification and renaturation of recombinant human interleukin-2. Biochem. J. 245: 85–91.
Itakura, K., Hirose, T., Crea, R., Riggs, A.D., Heyneker, H.L., Bolivar, F. and Boyer, H.W. 1977. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science 198: 1056–1063.
Shine, J., Fettes, I., Lan, N.C.Y., Roberts, J.L. and Baxter, J.D. 1980. Expression of cloned β-endorphin sequences by Escherichia coli. Nature 285: 456–461.
Adari, H.Y., Rose, K., Williams, K.R., Konisberg, W.H., Lin, T.-C. and Spicer, E.K. 1985. Cloning, nucleotide sequence, and overexpression of the bacteriophage T4 regA gene. Proc. Natl. Acad. Sci. USA 82: 1901–1905.
Sekine, S., Mizukami, T., Nishi, T., Kuwana, Y., Saito, A., Sato, M., Itoh, S. and Kawauchi, H. 1985. Cloning and expression of cDNA for the salmon growth hormone in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 82: 4306–4310.
Seeburg, P.H., Shine, J., Martial, J.A., Ivarie, R.D., Morris, J.E., Ultrich, A., Baxter, J.D. and Goodman, H. M. 1978. Cloning and expression of cDNA for bovine growth hormone in Escherichia coli. Nature 276: 795–798.
Szoka, P.R., Schreiber, A.B., Chan, H. and Murthy, J. 1986. A general method for retrieving the components of a genetically engineered fusion protein. DNA 5: 11–20.
White, J.H. and Richardson, C.C. 1988. Gene 19 of bacteriophage T7. Overexpression, purification, and characterization of its product. J. Biol. Chem. 263: 2469–2476.
Wetzel, R., Heyneker, H.L., Goeddel, D.V., Juhrani, P., Shapiro, J., Crea, R., Low, T.L., McClure, K., Thurman, J.E. and Goldstein, A.L. 1981. Production of biologically active Nα-desacetyl thymosin α in Escherichia coli through expression of a chemically synthesized gene, p. 251–266. In: Cellular Responses to Molecular Modulators. Mozes, L. W., Schultz, J., Scott, W. A., and Werner, R., (Eds.). Academic Press, New York.
Courtney, M., Buchwalder, A., Kohli, V., Lathe, R., Tolstoshev, P. and Lecocq, J.-P. 1984. High-level production of biologically active human α1-antitrypsin in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 81: 669–673.
Pennica, D., Hayflick, J.S., Bringman, T.S., Palladina, M.A. and Goeddel, D.V. 1985. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc. Natl. Acad. Sci. U.S.A. 82: 6060–6064.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilkinson, D., Harrison, R. Predicting the Solubility of Recombinant Proteins in Escherichia coli. Nat Biotechnol 9, 443–448 (1991). https://doi.org/10.1038/nbt0591-443
Issue Date:
DOI: https://doi.org/10.1038/nbt0591-443