Abstract
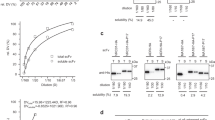
We have used a strategy of hybrid gene synthesis and constant domain shuffling to construct and functionally express in Escherichia coli genes encoding two anti–carbohydrate Fabs, one specific for a Brucella cell–surface polysaccharide and the second for the human blood group A determinant. Very similar VL amino acid sequences made possible the simultaneous synthesis of the two corresponding genes. A class switching approach was used in Fd and light chain gene assembly. The two independently synthesized VH genes were fused to a previously made sequence encoding the Cγ11 domain as an alternative to synthesis of the natural Cγ2b1 and Cμ1 sequences. The VL genes were initially coupled to a synthetic Cκ gene. When these light chain and the above Fd genes, each preceded by the ompA signal sequence, were expressed from two–cistron DNA, yields of functional periplasmic Fab were low and, in each instance, limited by light chain availability. Replacement of the Cκ domains with a Cλ1 domain resulted in a significant increase in the amount of soluble periplasmic light chain and functional Fab for both the Brucella and blood group A antibodies. The Cκ and Cλ1 forms of each of the Brucella and blood group A Fabs, with His5 fusions at the C–termini of the Fd chains, were purified by immobilized metal affinity chromatography. For the blood group A antibody, it was shown by ELISA that precise engineering of the elbow region was essential for full activity of the hybrid light chain constructs, since a two residue increase in elbow length abolished antigen binding activity. The Brucella antibody tolerated the longer elbow sequence. Sequences in the Cλ1 domain may result in increased yields of functional light chain by improving translocation across the cytoplasmic membrane or by reducing formation of periplasmic inclusion bodies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Better, M., Chang, C.P., Robinson, R.R. and Horwitz, A.H. 1988. Escherichia coli secretion of an active chimeric antibody fragment. Science 240: 1041–1043.
Skerra, A. and Plückthun, A. 1988. Assembly of a functional immunoglobulin FV fragment in Escherichia coli. Science 240: 1038–1040.
Anand, N.N., Mandal, S., MacKenzie, C.R., Sadowska, J., Sigurskjold, B., Young, N.M., Bundle, D.R. and Narang, S.A. 1991. Bacterial expression and secretion of various single-chain genes encoding proteins specific for a Salmonella serogroup B O-antigen. J. Biol. Chem. 266: 21874–21879.
McCafferty, J., Griffiths, A.D., Winter, G. and Chiswell, D.J. 1990. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348: 552–554.
Barbas, C.F., Kang, A.S., Lerner, R.A. and Benkovic, S.J. 1991. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc. Natl. Acad. Sci. USA 88: 7978–7982.
Anand, N.N., Dubuc, G., Phipps, J., MacKenzie, C.R., Sadowska, J., Young, N.M., Bundle, D.R. and Narang, S.A. 1991. Synthesis and expression in Escherichia coli of cistronic DNA encoding an antibody fragment specific for a Salmonella serogroup B O-antigen. Gene 100: 39–44.
Bundle, D.R., Cherwonogrodsky, J.W., Gidney, M.A.J., Meikle, P.J., Perry, M.B. and Peters, T. 1989. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect. Immun. 57: 2829–2836.
Chen, H.-T., Kabat, E.A., Lundblat, A. and Ratcliffe, R.M. 1987. Nucleotide and translated amino acid sequences of cDNA coding for the variable regions of the light and heavy chains of mouse hybridoma antibodies to blood group A and B substances. J. Biol. Chem. 262: 13579–13583.
Sung, W., Zahab, D.M., Yao, F.-L., Wu, R. and Narang, S.A. 1986. Simultaneous synthesis of human-, mouse- and chimeric epidermal growth factor genes via “hybrid gene synthesis” approach. Nucl. Acids Res. 14: 6159–6168.
Szybalski, W., Kim, S.C., Hasan, N. and Podhajska, A.J. 1991. Class-IIS restriction enzymes—a review. Gene 100: 13–26.
Narang, S.A., Yao, F.-L, Michniewicz, J.J., Dubuc, G., Phipps, I. and Somorjai, R.L. 1987. Hierarchial strategy for protein folding and design: synthesis and expression of T4 lysozyme gene and two putative mutants. Protein Eng. 1: 481–485.
Hoogenboom, H.R., Grifflths, A.D., Johnson, K.S., Chiswell, D.J., Hudson, P. and Winter, G. 1991. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucl. Acids Res. 19: 4133–4137.
Anand, N.N., Dubuc, G., Phipps, J., Gidney, M.A.J., Sinnott, B., Young, N.M., MacKenzie, C.R., Bundle, D.R. and Narang, S.A. 1990. Synthesis and expression in Escherichia coli of DNA encoding the murine λ1 chain of a monoclonal antibody specific for Salmonella serogroup B O-antigen. Protein Eng. 3: 541–546.
Skerra, A., Pfitzinger, I. and Plückthun, A. 1991. The functional expression of antibody FV fragments in Escherichia coli: Improved vectors and a generally applicable purification technique. Bio/Technology 9: 273–278.
Cygler, M., Rose, D.R. and Bundle, D.R. 1991. Recognition of a cell-surfece oligosaccharide of pathogenic Salmonella by an antibody Fab fragment. Science 253: 442–445.
Rose, D.R., Przybylska, M., To, R.J., Kayden, C. S., Oomen, R.P., Vorberg, E., Young, N.M. and Bundle, D.R. 1993. Crystal structure to 2.45 Å resolution of a monoclonal Fab specific for the Brucella A cell wall polysaccharide antigen. Protein Sci. 2: 1106–1113.
Stemmer, W.P.C., Morris, S.K., Kautzer, C.R. and Wilson, B.S. 1993. Increased antibody expression from Escherichia coli through wobble-base library mutagenesis by enzymatic inverse PCR. Gene 123: 1–7.
McManus, S. and Reichmann, L. 1991. Use of 2D NMR, protein engineering, and molecular modeling to study the hapten-binding site of an antibody FV fragment against 2-phenyloxazolone. Biochemistry 30: 5851–5857.
Brummell, D.A., Sharma, V.P., Anand, N.N., Bilous, D., Dubuc, G., Michniewicz, J.J., MacKenzie, C.R., Sadowska, J., Sigurskjold, B.W., Sinnott, B., Young, N.M., Bundle, D.R. and Narang, S.A. 1993. Probing the combining site of an anti-carbohydrate antibody by saturation mutagenesis: role of the heavy chain CDR3 residues. Biochemistry 32: 1180–1187.
Minsky, A., Summers, R.G. and Knowles, J.R. 1986. Secretion of β-lactamase into the periplasm of Escherichia coli: Evidence for a distinct release step associated with a conformational change. Proc. Natl. Acad. Sci. USA 83: 4180–4184.
Fitts, R., Reuveny, Z., van Amsterdam, J., Mulholland, J. and Botstein, D. 1987. Substitution of tyrosine for either cysteine in β-lactamase prevents release from the membrane during secretion. Proc. Natl. Acad. Sci. USA 84: 8540–8543.
Coleman, J., Inukai, M. and Inouye, M. 1985. Dual functions of the signal peptide in protein transfer across the membrane. Cell 43: 351–360.
Inouye, S., Wang, S., Sekizawa, J., Halegona, J. and Inouye, M. 1977. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc. Natl. Acad. Sci. USA 74: 1004–1008.
Plückthun, A. 1991. Strategies for the expression of antibody fragments in Escherichia coli. Methods: A Companion to Methodsin Enzymology 2: 88–96.
Skerra, A. and Plückthun, A. 1991. Secretion and in vivo folding of the Fab fragment of the antibody McPC603 in Escherichia coli: influence of disulphides and cis-prolines. Protein Eng. 4: 971–979.
Plückthun, A. 1992. Mono- and bivalent antibody fragments produced in Escherichia coli: engineering, folding and antigen binding. Immunol. Reviews 130: 151–188.
Glockshuber, R., Schmidt, T. and Plückthun, A. 1992. The disulfide bonds in antibody variable domains: effects on stability, folding in vitro, and functional expression in Escherichia coli. Biochemistry 31: 1270–1279.
Knappik, A., Krebber, C. and Plückthun, A. 1993. The effect of folding catalysts on the in vivo folding process of different antibody fragments expressed in Escherichia coli. Bio/Technology 11: 77–83.
Davies, D.R., Padlan, E.A. and Cohen, G.H. 1986. Fab assembly: an analysis of different CH1:CL combinations. Prog. Immunol. 6: 145–149.
Simon, T. and Rajewsky, K. 1990. Antigen domains mutants demonstrate autonomy of the antigen binding site. EMBO J. 9: 1051–1056.
Lesk, A.M. and Chothia, C. 1988. Elbow motion in the immunoglobulins involves a molecular ball-and-socket joint. Nature 335: 188–190.
Winter, G. and Milstein, C. 1991. Man-made antibodies. Nature 349: 293–299.
Oomen, R., Young, N.M. and Bundle, D.R. 1991. Molecular modelling of antibody-antigen complexes between the Brucella abortus O-chain polysaccharide and a specific monoclonal antibody. Protein Eng. 4: 427–433.
Wetzel, R., Perry, L.J. and Veilleux, C. 1990. Mutations in human interferon gamma affecting inclusion body formation identified by a general immunochemical screen. Bio/Technology 9: 731–737.
Sambrook, J., Fritsch, E.F. and Maniatis, T. Molecular Cloning: A Laboratory Manual (2nd ed.). Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MacKenzie, C., Sharma, V., Brummell, D. et al. Effect of Cλ–Cκ Domain Switching on Fab Activity and Yield in Escherichia Coli: Synthesis and Expression of Genes Encoding Two Anti–Carbohydrate Fabs. Nat Biotechnol 12, 390–395 (1994). https://doi.org/10.1038/nbt0494-390
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0494-390
This article is cited by
-
Facile domain rearrangement abrogates expression recalcitrance in a rabbit scFv
Applied Microbiology and Biotechnology (2015)