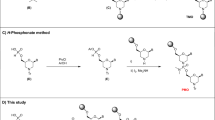
Abstract
Oligonucleoside methylphosphonates contain nonionic internucleotide bonds that resist degradation by cellular nucleases and allow the oligomers to be taken up intact by mammalian cells in culture. An-tisense methylphosphonate oligomers targeted against cellular or viral mRNA ini-tation codon or coding regions or against precursor mRNA splice sites effectively and specifically inhibit mRNA expression in cells. The efficacy of antisense methylphosphonate oligomers can be enhanced by derivatization with functional groups that allow the oligomer to covalently cross-link with its targeted mRNA. These oligonucleotide analogs will be useful tools for studying and controlling gene expression and are also promising candidates for development as therapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cohen, J.C. (Ed.). 1989. Oligodeoxyribonucleotides. Antisense Inhibitors of Gene Expression. Topics in Molecular and Structural Biology 12. MacMillan Press, London.
Cooney, M., Czernuszewicz, G., Postel, E.H., Flint, S.J. and Hogan, M. 1988. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science 241: 456–464.
Francois, J.C., Sainson-Behmoaras, T. and Helene, C. 1988. Sequence-specific recognition of the major groove of DNA by oligode-oxynucleotides via triple helix formation. Footprinting studies. Nucleic Acids Res. 16: 11431–11440.
Povsic, T.J. and Dervan, P.B. 1989. Triple helix formation by oligonucleotides on DNA extended to the physiological pH range. J. Am. Chem. Soc. 111: 3059–3061.
Maher, III, L.J., Wold, B. and Dervan, P.B. 1989. Inhibition of DNA binding proteins by oligonucleotide-directed triple helix formation. Science 245: 725–730.
Griffin, L.C. and Dervan, P.B. 1989. Recognition of thymine-adenine base pairs by guanine in a pyrimidine triple helix motif. Science 245: 967–971.
Corey, D., Pei, D. and Schultz, P. 1989. Sequence-selective hydrolysis of duplex DNA by an oligonucleotide-directed nuclease. J. Amer. Chem. Soc. 111: 8523–8525.
Francois, J.-C., Saison-Behmoaras, T., Barbier, C., Chassignol, M., Thuong, N. and Helene, C. 1989. Sequence-specific recognition and cleavage of duplex DNA via triple-helix formation by oligonucleotides covalently linked to a phenanthroline-copper chelate. Proc. Natl. Acad. Sci. USA 86: 9702–9706.
Sun, J.-S., Francois, J.-C., Montenay-Garestier, T., Saison-Behmoaras, T., Roig, V., Thuong, N. and Helene, C. 1989. Sequence-specific intercalating agents: Intercalation at specific sequences on duplex DNA via major groove recognition by oligonucleotide-intercalator conjugates. Proc. Natl. Acad. Sci. USA. 86: 9198–9202.
Francois, J.-C., Saison-Behmoaras, T., Thuong, N. and Helene, C. 1989. Inhibition of restriction endonuclease cleavage via triple helix formation by homopyrimidine oligonucleotides. Biochemistry 28: 9617–9619.
Strobel, S. and Dervan, P. 1990. Site-specific cleavage of a yeast chromosome by oligonucleotide-directed triple-helix formation. Science 249: 73–75.
Horne, D. and Dervan, P. 1990. Recognition of mixed-sequence duplex DA by alternate-strand triple-helix formation. J. Am. Chem. Soc. 112: 2435–2437.
Perrouault, L., Asseline, U., Rivalle, C., Thuong, N.T., Bisagni, E., Giovannangeli, C., Le Doan, T. and Helene, C. 1990. Sequence-specific artificial photoinduced endonucleases based on triple helix-forming oligonucleotides. Nature 344: 359–360.
Birg, F., Preseuth, D., Zerial, A., Thuong, N.T., Asseline, U., Le Doan, T. and Helene, C. 1990. Inhibition of simian virus 40 DNA replication in CV-1 cells by an oligodeoxynucleotide covalently linked to an intercalating agent. Nucleic Acids Res. 18: 2901–2908.
Helene, C. and Toulme, J.-J. 1989. Control of gene expression by oligodeoxynucleotides covalently linked to intercalating agents and nucleic acid-cleaving reagents, p. 135–172. In: Oligodeoxyribonucleotides. Antisense Inhibitors of Gene Expression. J. S. Cohen (Ed.). Topics in Molecular and Structural Biology 12. MacMillan Press, London.
Miller, P.S. and Ts'o, P.O.P. 1988. Oligonucleotide inhibitors of gene expression in living cells: New opportunities in drug design. Annual Reports Med. Chem. 23: 295–304.
Miller, P.S. 1990. Antisense nucleic acid analogues as potential antiviral agents, p. 343–360. In: Herpesvirus, the Immune System and AIDS. L. Aurelian (Ed.). Kluwer Academic Publishers, Boston.
Uhlmann, E. and Peyman, A. 1990. Antisense oligonucleotides: A new therapeutic principle. Chem. Rev. 90: 544–579.
Stein, C., Mori, K., Loke, S., Subasinghe, C., Shinozuka, K., Cohen, J. and Neckers, L. 1988. Phosphorothioate and normal oligodeoxyribo-nucleotides with 5′-linked acridine: characterization and preliminary kinetics of cellular uptake. Gene 72: 333–341.
Loke, S., Stein, C., Zhang, X., Mori, K., Nakanishi, M., Subasinghe, C., Cohen, J. and Neckers, L. 1989. Characterization of oligonucleotide transport into living cells. Proc. Natl. Acad. Sci. USA 86: 3474–3478
Yakubov, L., Deeva, E., Zarytova, V., Ivanova, E., Ryte, A., Yurch-enko, L. and Vlassov, V. 1989. Mechanism of oligonucleotide uptake by cells: Involvement of specific receptors? Proc. Natl. Acad. Sci. USA 86: 6454–6458.
Cazenave, C., Chevrier, M., Thuong, N.T. and Helene, C. 1987. Rate of degradation of alpha- and beta-oligodeoxynucleotides in Xenopus oocytes. Implications for anti-messenger strategies. Nucleic Acids Res. 15: 10507–10521.
Jessus, C., Cazenave, C., Ozon, R. and Helene, C. 1988. Specific inhibition of endogenous beta-tubulin synthesis in Xenopus oocytes by anti-messenger oligodeoxynucleotides. Nucleic Acids Res. 16: 2225–2233.
Shuttleworth, J. and Colman, A. 1988. Antisense oligonucleotide-directed cleavage of mRNA in Xenopus oocytes and eggs. EMBO J. 7: 427–434.
Saxena, S. and Ackerman, E. 1990. Microinjected oligonucleotides complementary to the alpha-sarcin loop of 28 S RNA abolish protein synthesis in Xenopus oocytes. J. Biol. Chem. 265: 3263–3269.
Blondel, D., Harmison, G. and Schubert, M. 1990. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J. Virol. 64: 1716–1725.
Woolf, T.M., Jennings, C.G.B., Rebagliati, M. and Melton, D.A. 1990. The stability, toxicity and effectiveness of unmodified and phosphorothioate antisense oligodeoxynucleotides in Xenopus oocytes and embryos. Nucleic Acids Res. 18: 1763–1769
Miller, P.S. 1989. Non-ionic antisense oligonucleotides, p. 79–95. In: Oligodeoxyribonucleotides. Antisense Inhibitors of Gene Expression. J. S. Cohen (Ed.). Topics in Molecular and Structural Biology 12. MacMillan Press, London.
Matteucci, M. 1990. Deoxyoligonucleotide analogs based on formace-tal linkages. Tetrahedron Letters 17: 2385–2388.
Droman, M.A., Noble, S.A., McBride, L.J. and Caruthers, M.H. 1984. Synthesis of oligonucleotides and oligodeoxynucleotide analogs using phosphoramidite intermediates. Tetrahedron 49: 95–102.
Jager, A. and Engels, J. 1984. Synthesis of deoxynucleoside methylphos-phonates via a phosphonamidite approach. Tetrahedron Letters 25: 1437–1440.
Stec, W.J., Zon, G., Egan, W., Byrd, R.A., Phillips, L.R. and Gallo, K.A. 1985. Solid-phase synthesis, separation and stereochemical aspects of P-chiral methane-and 4,4′-dimethoxytriphenyl-methanephos-phonate analogues of Oligodeoxyribonucleotides. J. Org. Chem. 50: 3908–3913.
Agrawal, S. and Goodchild, J. 1987. Oligodeoxynucleoside methylphosphonates: synthesis and enzymic degradation. Tetrahedron Letters 28: 3539–3542.
Murakami, A., Blake, K.R. and Miller, P.S. 1985. Characterization of sequence-specific oligodeoxyribonucleoside methylphosphonates and their interaction with rabbit globin mRNA. Biochemistry 24: 4041–4046.
Lee, B.L., Murakami, A., Blake, K.R. and Miller, P.S 1988. Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with single-stranded DNA. Biochemistry 27: 3197–3203.
Lee, B.L., Blake, K.R. and Miller, P.S. 1988. Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with synthetic DNA containing a promoter for T7 RNA polymerase. Nucleic Acids Res. 16: 10681–10697.
Kean, J.M., Murakami, A., Blake, K.R., Cushman, C.D. and Miller, P.S. 1988.Photochemical cross-linking of psoralen-derivatized oligonucleoside methylphosphonates to rabbit globin messenger RNA. Biochemistry 27: 9113–9121.
Bhan, P. and Miller, P.S. 1990. Photo cross-linking of psoralen derivatized oligonucleoside methylphosphonates to single stranded DNA. Bioconjugate Chemistry 1: 82–88.
Amirkhanov, N. and Zarytova, F. 1989. Reactive oligonucleotides bearing methylphosphonate groups. III. Affinity modification of nucleic acid target by 4-(N-2-chloroethyl-N-methylamino)benzyl-3′-and 5′-phosphoamide derivatives having stereoregular methylphosphonate residues. Bioorganicheskaya Khimiya 15: 379–385.
Lin, S.-B., Blake, K.R., Miller, P.S. and Ts'o, P.O.P. 1989. Use of EDTA derivatization to characterize interactions between oligodeoxyribonucleoside methylphosphonates and nucleic acids. Biochemistry 28: 1054–1061.
Froehler, B. and Matteucci, M. 1988. Phosphoramidate analogues of DNA: synthesis and thermal stability of heteroduplexes. Nucleic Acids Res. 16: 4831–4839.
Sarin, P.S., Agrawal, S., Civeira, M.P., Goodchild, J., Ikeuchi, T. and Zamecnik, P.C. 1988. Inhibition of acquired immunodeficiency syndrome virus by oligodeoxynucleoside methylphosphonates. Proc. Natl. Acad. Sci. USA 85: 7448–7451.
Quartin, R.S. and Wetmur, J.G. 1989. Effect of ionic strength on the hybridization of oligodeoxynucleotides with reduced charge due to methylphosphonate linkages to unmodified oligodeoxynucleotides containing the complementary sequence. Biochemistry 28: 1040–1047.
Miller, P.S., Dreon, N., Pulford, S.M. and McParland, K.B. 1980. Oligothymidylate analogues having stereoregular, alternating meth-ylphosphonate/phosphodiester backbones. Synthesis and physical studies. J. Biol. Chem. 235: 9659–9665.
Lesnikowski, Z.J., Jaworska, M. and Stec,W.J. 1990. Octa(thymidine methanephosphonates) of partially defined stereochemistry: synthesis and effect of chirality at phosphorus on binding to pentadecadeoxyri-boadenylic acid. Nucleic Acids Res. 18: 2109–2115.
Miller, P.S., Bhan, P., Cushman, C.D., Kean, J.M. and Levis, J.T. 1990. Antisense oligonucleoside methylphosphonates and their derivatives. Nucleosides and Nucleotides In press.
Miller, P.S., McParland, K.B., Jayaraman, K. and Ts'o, P.O.P. 1981. Biochemical and biological effects of nonionic nucleic acid methylphosphonates. Biochemistry 20: 1874–1880.
Marcus-Sekura, C.J., Woerner, A.M., Shinozuka, K., Zon, G. and Quinnan, G.V., Jr. 1987. Comparative inhibition of chloramphenicol acetyltransferase gene expression by antisense oligonucleotide analogues having alkyl phosphotriester, methylphosphonate and phos-phorothioate linkages. Nucleic Acids Res. 15: 5749–5763.
Vasanthakumar, G. and Ahmed, N.K. 1989. Modulation of drug resistance in a daunorubicin resistance subline with oligonucleoside methylphosphonates. Cancer Communications 1: 225–232.
Jayaraman, K., McParland, K., Miller, P. and Ts'o, P.O.P. 1981. Selective inhibition of Escherichia coli protein synthesis and growth by nonionic oligonucleotides complementary to the 3′ end of 16S rRNA. Proc. Natl. Acad. Sci. USA 78: 1537–1541.
Blake, K.R., Murakami, A., Spitz, S.A., Glave, S.A., Reddy, M.P., Ts'o, P.O.P. and Miller, P.S. 1985. Hybridization arrest of globin synthesis in a rabbit reticulocyte lysate and in rabbit reticulocytes by oligodeoxyribonucleoside methylphosphonates. Biochemistry 24: 6139–6145.
Agris, C.H., Blake, K.R., Miller, P.S., Reddy, M.P. and Ts'o, P.O.P. 1986. Inhibition of vesicular stomatitis virus protein synthesis and infection by sequence-specific oligodeoxyribonucleoside methylphosphonates. Biochemistry 25: 6268–6275.
Yu, Z., Chen, D., Black, R.J., Blake, K., Ts'o, P.O.P., Miller, P. and Chang, E.H. 1989. Sequence specific inhibition of in vitro translation of mutated or normal ras p21. J. Experimental Pathology 4: 97–108.
Maher, L.J., III and Dolnick, B.J. 1987. Specific hybridization arrest of dihydrofolate reductase mRNA in vitro using anti-sense RNA or anti-sense oligonucleotides. Arch. Biochem. Biophys. 253: 214–220.
Walder, R.Y. and Walder, J.A. 1988. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc. Natl. Acad. Sci. USA 85: 5011–5015.
Quartin, R., Brakel, C. and Wetmur, J. 1989. Number and distribution of methylphosphonate linkages in oligodeoxynucleotides affect exo- and endonuciease sensitivity and ability to form RNase H substrates. Nucleic Acids Res. 17: 7253–7262.
Furdon, P., Dominski, Z. and Kole, R. 1989. RNase H cleavage of RNA hybridized to oligonucleotides containing methylphosphonate, phosphorothioate and phosphodiester bonds. Nucleic Acids Res. 17: 9193–9204.
Agrawal, S., Mayrand, S., Zamecnik, P. and Pederson, T. 1990. Site-specific excision from RNA by RNase H and mixed-phosphate-backbone oligodeoxynucleotides. Proc. Natl. Acad. Sci. USA 87: 1401–1405.
Miller, P.S. 1991. Antisense oligonucleoside methylphosphonates. In: Antisense RNA and DNA J. A. H. Murray (Ed.). J. Wiley and Sons, New York.
Miller, P.S., Agris, C.H., Aurelian, L., Blake, K.R., Murakami, A., Reddy, M.P., Spitz, S.A. and Ts'o, P.O.P. 1985. Control of ribonu-cleic acid function by oligonucleoside methylphosphonates. Biochimie 67: 769–776.
Smith, C.C., Aurelian, L., Reddy, M.P., Miller, P.S. and Ts'o, P.O.P. 1986. Antiviral effect of an oligo (nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc. Natl. Acad. Sci. USA 83: 2787–2791.
Kulka, M., Smith, C., Aurelian, L., Fishelevich, R., Meade, K., Miller, P. and Ts'o, P.O.P. 1989. Site specificity of the inhibitory effects of oligo(nucleoside methylphosphonates) complementary to the acceptor splice junction of herpes simplex virus type 1 immediate early mRNA 4. Proc. Natl. Acad. Sci. USA 86: 6868–6872.
Zaia, J.J., Rossi, J.J., Murakawa, G.J., Spallone, P.A, Stephens,D.A., Kaplan,R.E., Eritja,R., Wallace, R.B. and Cantin,E.M. 1988. Inhibition of human immunodeficiency virus by using an oligonucleoside methylphosphonate targeted to the tat-3 gene. J. Virol. 62: 3914–3917.
Brown, D., Yu, Z., Miller, P., Blake, K., Wei, C., Black, R., Ts'o, P. and Chang, E. 1989. Modulation of ras expression by anti-sense, nonionic deoxyoligonucleotide analogs. Oncogene Research 4: 243–252.
Chen,T.-L., Miller,P.S., Ts'o, P.O.P. and Colvin,O.M. 1990. Disposition and metabolism of oligodeoxynucleoside methylphosphonate following a single IV injection in mice. Drug Metabolism and Disposition. In press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miller, P. Oligonucleoside Methylphosphonates as Antisense Reagents. Nat Biotechnol 9, 358–362 (1991). https://doi.org/10.1038/nbt0491-358
Issue Date:
DOI: https://doi.org/10.1038/nbt0491-358
This article is cited by
-
A Drosera-bioinspired hydrogel for catching and killing cancer cells
Scientific Reports (2015)
-
Oligonucleoside Methylphosphonates as Antisense Reagents
Nature Biotechnology (1991)