Abstract
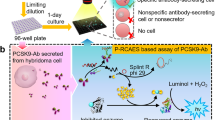
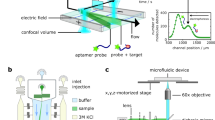
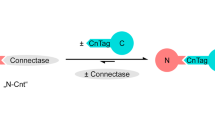
Protein-protein and protein-peptide interactions that are low affinity in nature preclude the straightforward measurement of binding. To overcome this limitation, a novel method has been devised for stabilizing these weak interactions by increasing the binding avidity. These studies have focused on the binding of peptides to heat shock proteins (with a typical KD of approximately 25 to 50 μM). Multivalent ligands have been created by coupling peptides plus biotin to a neutral carrier molecule, dex-tran. These peptide-dextran conjugates allow for more avid binding to proteins that have been immobilized on a membrane surface. Detection of signals via enhanced chemiluminescence further increases the sensitivity of the method that has been termed Chemiluminescence of Enhanced Avidity Reactions (CLEAR). The assay is simple, reliable and consistently detects specific binding between heat shock proteins and peptide ligands. CLEAR should be generally applicable to other ligand receptor pairs where the detection of binding is limited by the low affinity of the interaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Anathan, J., Goldberg, A.L. and Voellmy, R. 1986. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science 232: 522–524.
Gething, M.J. and Sambrook, J. 1992. Protein folding in the cell. Nature 365: 33–45.
Landry, S.J. and Gierasch, L.M. 1991. Recognition of nascent polypeptides for targeting and folding. Trends Biochem. Sci. 16: 159–163.
Shi, Y. and Thomas, J.O. 1992. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol. Cell Biol. 12: 2186–2192.
Dice, J.F. 1990. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem. Sci. 15: 305–309.
Terlecky, S.R. Hsp70s and lysosomal proteolysis. 1994. Experientia 50: 1021–1025.
Flynn, G.C., Chappell, T.G. and Rothman, J.E. 1989. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science 245: 385–390.
Flynn, G.C., Pohl, J., Flocco, M.T. and Rothman, J.E. 1991. Peptide-binding specificity of the molecular chaperone BiP. Nature 353: 726–730.
Landry, S.J. and Gierasch, L.M. 1991. The chaperonin GroEL binds a polypep-tide in an α-helical conformation. Biochemistry 30: 7359–7362.
Landry, S.J., Jordan, R., McMacken, R. and Gierasch, L.M. 1992. Different conformations for the same polypeptide bound to chaperones DnaK and GroEL. Nature 355: 455–457.
Schmid, D., Baici, A., Gehring, H. and Christen, P. 1994. Kinetics of molecular chaperone action. Science 263: 971–973.
Terlecky, S.R., Chiang, H.L., Olson, T.S. and Dice, J.F. 1992. Protein and peptide binding and stimulation of in vitro lysosomal proteolysis by the 73-kDa heat shock cognate protein. J. Biol. Chem. 267: 9202–9209.
Nadeau, K., Nadler, S.G., Saulnier, M., Tepper, M.A. and Walsh, C.T. 1994. Quantitation of the interaction of the immunosuppressant deoxyspergualin and analogs with Hsc70 and Hsp90. Biochem. 33: 2561–2567.
Karush, F. 1976. Multivalent binding and functional affinity. Contemp. Top. Mol. Immunol. 5: 217–228.
Goodwin, D.A., Meares, C.F., McTigue, M., Chaovapong, W., Diamanti, C.I., Ransone, C.H. and McCall, M.J. 1992. Pretargeted immunoscintigraphy: effect of hapten valency on murine tumor uptake. J. Nucl. Med. 33: 2006–2013.
Arulanandam, A.R.N., Moingeon, P., Concino, M.F., Recny, M.A., Kato, K., Yagita, H., Koyasu, S. and Reinherz, E.L. 1993. A soluble multimeric recombinant CD2 protein identifies CD48 as a low affinity ligand for human CD2: divergence of CD2 ligands during the evolution of humans and mice. J. Exp. Med. 177: 1439–1450.
Parish, C.R., Recny, M.A., Knoppers, M.H., Waldron, J.C. and Warren, H.S. 1993. Detection of a glycosylation-dependent ligand for the T lymphocyte cell adhesion molecule CD2 using a novel multimeric recombinant CD2-binding assay. J. Immunol. 150: 4833–4843.
So/ndergård-Andersen, J., Lauritzen, E., Lind, K. and Holm, A. 1990. Covalently linked peptides for enzyme-linked immunosorbent assay. J. Immunol. Meth. 131: 99–104.
Fayet, O., Ziegelhoffer, T. and Georgopoulos, C. 1988. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J. Bacteriol. 171: 1379–1385.
Rippmann, R., Taylor, W.R., Rothbard, J.B. and Green, N.M. 1991. A hypothetical model for the peptide binding domain of hsp70 based on the peptide binding domain of HLA. EMBO J. 10: 1053–1059.
Flaherty, K.M., Deluca-Flaherty, C. and McKay, D.B. 1990. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature 346: 623–628.
Flaherty, K.M., McKay, D.B., Kabsch, W. and Holmes, K.C. 1991. Similarity of the three-dimensional structures of actin and the ATPase fragment of a 70-kDa heat shock cognate protein. Proc. Natl. Acad. Sci. USA 88: 5041–5045.
Moss, M.L., Palmer, R.E., Kuzmic, P., Dunlap, B.E., Henzel, W., Kofron, J.L., Mellon, W.S., Royer, C.A. and Rich, D.H. 1992. Identification of actin and Hsp70 as cyclosporin A binding proteins by photoaffinity labeling and fluorescence displacement assays. J. Biol. Chem. 267: 22054–22059.
Nadler, S.G., Cleaveland, J., Tepper, M.A., Walsh, C. and Nadeau, K. 1993. Studies on the interaction of the immunosuppressant 15-deoxyspej:gualin with heat shock proteins. Ann. N.Y. Acad. Sci. 685: 412–414.5.
Lowry, O.H., Roseborough, N.J., Farr, A.L. and Randall, R.J. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193: 265–275.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Causey, L., Dwyer, D. Detection of Low Affinity Interactions Between Peptides and Heat Shock Proteins by Chemiluminescence of Enhanced Avidity Reactions (CLEAR). Nat Biotechnol 14, 348–351 (1996). https://doi.org/10.1038/nbt0396-348
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0396-348
This article is cited by
-
Nucleic acid aptamers for clinical diagnosis: cell detection and molecular imaging
Analytical and Bioanalytical Chemistry (2011)