Abstract
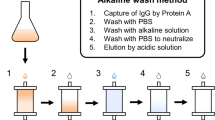
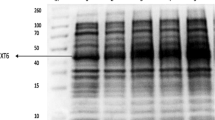
We have used an expanded bed adsorption procedure for efficient recovery of a recombinant fusion protein, directly from a crude fermentor broth without prior cell removal. The fusion protein was designed to have a relatively low isoelectric point (pI) to allow anionic exchange adsorption at pH 5.5 where most Escherichia coli host proteins are not adsorbed. The gene product was secreted to the culture medium of the E. coli host cells in high yields (550 mg/l). The separation of cells and the concentration and recovery of the fusion protein could therefore be achieved by a single unit operation. The yield after the expanded bed adsorption exceeded 90 percent. Furthermore, the significant volume reduction by the expanded bed adsorption, enabled efficient and straight–forward polishing of the product by a subsequent affinity chromatography step, for removal of contaminating DNA and pyrogenic compounds to levels acceptable for regulatory authorities. An overall yield exceeding 90 percent was maintained after the affinity chromatography polishing step. The procedure outlined here is suitable for large–scale bioprocesses and allows efficient removal of cells, host proteins, contaminating DNA and endotoxins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Datar, R.V.,, Cartwright, T. and Rosen, C.-G. 1993. Process economics of animal cell and bacterial fermentations: a case study analysis of tissue plasminogen activator. Bio/Technol. 11: 349–357
Shuster, J.R. 1991. Gene expression in yeast: protein secretion. Curr. Opin. Biotechnol. 2: 685–690
Kumar, V., Ramakrishnan, S., Teeri, T.T., Knowles, J.K.C. and Hartley, B.S. 1992. Saccaromyces cerevisiae cells secreting an Aspergillus niger β-galactosidase grown on whey permeate. Bio/Technology 10: 82–85
Abrahmsén, L., Moks, T., Nilsson, B. and Uhlén, M. 1986. Secretion of heterologous gene products to the culture medium of Escherichia coli. Nucl. Acids Res. 14: 7487–7500.
Suominen, I., Karp, M., Lähde, M., Kopio, A., Glumoff, T., Meyer, P. and Mäntsälä, P. 1987. Extracellular production of cloned α-amylase by Escherichia coli. Gene 61: 165–176.
Moks, T., Abrahmsén, L., Österlöf, B., Josephson, S., Östling, M., Enfors, S.-O., Persson, I., Nilsson, B. and Uhlén, M. 1987. Large-scale affinity purification of human insulin-like growth factor I from culture medium of Escherichia coli. Bio/Technology 5: 379–382.
Kitai, K., Kudo, T., Nakamura, S., Masegi, T., Ichikawa, Y. and Horikoshi, K. 1988. Extracellular production of human immunoglobulin G Fc region (hIgG-Fc) by Escherichia coli. Appl. Microbiol. Biotechnol. 28: 52–56.
Obukowicz, M.G., Turner, M.A., Wong, E.Y. and Tacon, W.C. 1988. Secretion and export of IGF-1 in Escherichia coli strain JM101. Mol. Gen. Genet. 215: 19–25.
Thomas, W.D. Jr., Wagner, S.P. and Welch, R.A. 1992. A heterologous membrane protein domain fused to the C-terminal domain of HlyB can export Escherichia coli hemolysin. J. Bacteriol. 174: 771–779.
Nilsson, B., Abrahmsén, L. and Uhlén, M. 1985. Immobilization and purification of enzymes with staphylococcal protein A gene fusion vectors. EMBO J. 4: 1075–1080.
Ward, E.S., Güssow, D., Griffiths, A.D., Jones, P.T. and Winter, G. 1989. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature 341: 544–546.
Carter, P., Kelley, R.F., Rodrigues, M.L., Snedecor, B., Covarrubias, M., Velligan, M.D., Wong, W.L.T., Rowland, A.M., Kotts, C.E., Carver, M.E., Yang, M., Bourell, J.H., Shepard, H.M. and Henner, D. 1992. High level Escherichia coli expression and production of a bivalent humanized antibody fragment. Bio/Technology 10: 163–167.
Draeger, N.M. and Chase, H.A. 1991. Liquid fluidized bed adsorption of protein in the presence of cells. Bioseparation 2: 67–80.
Chase, H.A. and Draeger, N.M. 1992. Affinity purification of proteins using expanded beds. J. Chromatogr. 597: 129–145.
Terranova, B.E. and Burns, M.A. 1991. Continous cell suspension processing using magnetically stabilized fluidized beds. Biotechnol. Bioeng. 37: 110–120.
Buijs, A. and Wesselingh, J.A. 1980. Batch fluidized ion-exchange column for streams containing suspended particles. J. Chromatogr. 201: 319–327.
Nilsson, B., Moks, T., Jansson, B., Abrahmsén, L., Elmblad, A., Holmgren, E., Henrichson, C., Jones, T.A. and Uhlén, M. 1987. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Engineering 1: 107–113.
Sjölander, A., Hansson, M., Lövgren, K., Wåhlin, B., Berzins, K. and Perlmann, P. 1993. Immunogenicity in rabbits and monkeys of influenza ISCOMs conjugated with repeated sequences of the Plasmodium falciparum antigen Pfl55/RESA. Parasite Immunol. 15: 355–359.
Favaloro, J.M., Coppel, R.L., Corcoran, L.M., Foote, S.J., Brown, G.V., Anders, R.F. and Kemp, D.J. 1986. Structure of the RESA gene of Plasmodium falcipanm. Nucl. Acids Res. 14: 8265–8277.
Sjölander, A., Ståhl, S. and Perlmann, P. 1993. Bacterial expression systems based on protein A and protein G designed for the production of immunogens: applications to Plasmodium falciparum malaria antigens. Immunomethods 2: 79–92.
Uhlén, M. and Moks, T. 1990. Gene fusions for purpose of expression: an introduction. Methods Enzymol. 185: 129–143.
Ståhl, S., Sjölander, A., Nygren, P.-Å., Berzins, K., Perlmann, P. and Uhlén, M. 1989. A dual expression system for the generation, analysis and purification of antibodies to a repeated sequence of the Plasmodium falciparum malaria antigen Pfl155/RESA J. Immunol. Meth. 124: 43–52.
Ståhl, S., Sjölander, A., Hansson, M., Nygren, P.-Å. and Uhlén, M. 1990. A general strategy for polymerization, assembly and expression of epitope-carrying peptides applied to the Plasmodium falciparum malaria antigen Pfl55/ RESA. Gene 89: 187–193.
Sjölander, A., Lövgren, K., Ståhl, S., Åslund, L., Hansson, M., Nygren, P.-A., Larsson, M., Hagstedt, M., Wåhlin, B., Berzins, K., Uhlén, M., Morein, B. and Perlmann, P. 1991. High antibody responses in rabbits immunized with influenza virus ISCOMs containing a repeated sequence of the Plasmodium falciparum malaria antigen Pfl55/RESA. Vaccine 9: 443–450.
Maurer, R., Meyer, B.J. and Ptashne, M. 1980. Gene regulation at the right operator (OR) of bacteriophage λ I OR3 and autogenous negative control by represser. J. Mol. Biol. 139 147–161.
Köhler, K., Ljungquist, C., Kondo, A., Veide, A. and Nilsson, B. 1991. Engineering proteins to enhance their partition coefficients in aqueous two-phase systems. Bio/Technology 9: 642–646.
Kung, V.T., Panfili, P.R., Sheldon, E.L., King, R.S., Nagainis, P.A., Gomez, B. Jr., Ross, D.A., Briggs, J. and Zuk, R.F. 1990. Picogram quantitation of total DNA using DNA-binding proteins in a silicon sensor-based system. Anal. Biochem. 187: 220–227.
Bacterial endotoxins test, 85, United States Pharmacopeia XXII.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hansson, M., Ståhl, S., Hjorth, R. et al. Single–Step Recovery of a Secreted Recombinant Protein by Expanded Bed Adsorption. Nat Biotechnol 12, 285–288 (1994). https://doi.org/10.1038/nbt0394-285
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0394-285
This article is cited by
-
Fabrication and characterisation of a novel pellicular adsorbent customised for the effective fluidised bed adsorption of protein products
Biotechnology and Bioprocess Engineering (2001)
-
Expanded-bed adsorption utilizing ion-exchange resin to purify extracellular β-galactosidase
Applied Biochemistry and Biotechnology (1998)