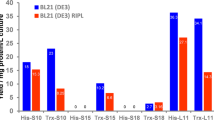
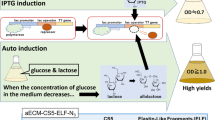
Abstract
We have developed a versatile Escherichia coli expression system based on the use of E. coli thioredoxin (trxA) as a gene fusion partner. The broad utility of the system is illustrated by the production of a variety of mammalian cytokines and growth factors as thioredoxin fusion proteins. Although many of these cytokines previously have been produced in E. coli as insoluble aggregates or “inclusion bodies”, we show here that as thioredoxin fusions they can be made in soluble forms that are biologically active. In general we find that linkage to thioredoxin dramatically increases the solubility of heterologous proteins synthesized in the E. coli cytoplasm, and that thioredoxin fusion proteins usually accumulate to high levels. Two additional properties of E. coli thioredoxin, its ability to be specifically released from the E. coli cytoplasm by osmotic shock or freeze/thaw treatments and its intrinsic thermal stability, are retained by some fusions and provide convenient purification steps. We also find that the active-site loop of E. coli thioredoxin can be used as a general site for small peptide insertions, allowing for the high level production of soluble peptides in the E. coli cytoplasm.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Stormo, G.D., Schneider, T.D. and Gold, L. 1982. Characterization of translation initiation sites in E. coli. Nucl. Acids Res. 10: 2971–2996.
Gottesman, S. 1989. Genetics of proteolysis in Escherichia coli. Annu. Rev. Genet. 23: 163–198.
Schein, C.H. 1989. Production of soluble recombinant proteins in bacteria. Bio/Technology 7: 1141–1149.
Mitraki, A. and King, J. 1989. Protein folding intermediates and inclusion body formation. Bio/Technology 7: 690–697.
Carter, P., Kelley, R.F., Rodrigues, M.L., Snedecor, B., Covarrubias, M., Velligan, M.D., Wong, W.L.T., Rowland, A.M., Kotts, C.E., Carver, M.E., Yang, M., Bourell, J.H., Shepard, H.M. and Henner, D. 1992. High level Escherichia coli expression and production of a bivalent humanized antibody fragment. Bio/Technology 10: 163–167.
Stader, J.A. and Silhavy, T.J. 1990. Engineering Escherichia coli to secrete heterologous gene products. Methods in Enzymol. 165: 166–187.
Georgiou, G., Telford, J.N., Shuler, M.L. and Wilson, D.B. 1986. Localization of inclusion bodies in Escherichia coli overproducing β-lactamase or alkaline phosphatase. Appl. Environ. Microbiol. 52: 1157–1161.
Bowden, G.A. and Giorgiou, G. 1990. Folding and aggregation of β-lactamase in the periplasmic space of Esherichia coli. J. Biol. Chem. 265: 16760–16766.
Ruther, U. and Muller-Hill, B. 1983. Easy identification of cDNA clones. EMBO J. 2: 1791–1794.
Yansura, D.G. 1990. Expression as trpE fusion. Methods in Enzymol. 165: 161–166.
Nilsson, B., Abrahmsen, L. and Uhlen, M. 1985. Immobilization and purification of enzymes with staphylococcal protein A gene fusion vectors. EMBO J. 4: 1075–1080.
Smith, D.B. and Johnson, K.S. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione-S-transferase. Gene 67: 31–40.
Maina, C.V., Riggs, P.D., Grandea, A.G., Slatko, B.E., Moran, L.S., Tagliamonte, J.A., McReynolds, L.A. and di Guan, C. 1988. An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from maltose-binding protein. Gene 74: 365–373.
Holmgren, A. 1985. Thioredoxin. Ann. Rev. Biochem. 54: 237–271.
Lunn, C.A., Kathju, S., Wallace, B.J., Kushner, S.R. and Pigiet, V. 1984. Amplification and purification of plasmid-encoded thioredoxin from Escherichia coli K12. J. Biol. Chem. 259: 10469–10474.
Katti, S.K., LeMaster, D.M. and Eklund, H. 1990. Crystal structure of thioredoxin from Escherichia coli at 1.68 angstroms resolution. J. Mol. Biol. 212: 167–184.
Bayer, M.E. 1968. Areas of adhesion between wall and membrane of Escherichia coli. J. Gen. Microbiol. 53: 395–404.
Lunn, C.A. and Pigiet, V.P. 1982. Localization of thioredoxin from Escherichia coli in an cosmotically sensitive compartment. J. Biol. Chem. 257: 11424–11430.
Tsuji, T., Nakagawa, R., Sugimoto, N. and Fukuhara, K.-I. 1987. Characterization of disulfide bonds in recombinant proteins: reduced human interleukin 2 in inclusion bodies and its oxidative refolding. Biochemistry 26: 3129–3134.
Donahue, R.E., Seehra, J., Metzger, M., Lefebvre, D., Rock, B., Carbone, S., Nathan, D.G., Garnick, M., Sehgal, P.K., Laston, D., LaVallie, E., McCoy, J., Schendel, P., Norton, C., Turner, K., Yang, Y.-C. and Clark, S.C. 1988. Human IL-3 and GM-CSF act synergistically in stimulating hematopoiesis in primates. Science 241: 1820–1823.
van Kimmenade, A., Bond, M.W., Schumacher, J.H., Laquoi, C. and Kastelein, R. A. 1988. Expression, renaturation and purification of recombinant human interleukin 4 from Escherichia coli. Eur. J. Biochem. 173: 109–114.
Arcone, R., Pucci, P., Zapacosta, F., Fontaine, V., Malorni, A., Marino, G. and Ciliberto, G. 1991. Single-step purification and structural characterization of human interleukin-6 produced in Escherichia coli from a T7 RNA polymerase expression vector. Eur. J. Biochem. 198: 541–547.
Martin, F.H., Suggs, S.V., Langley, K.E., Lu, H.S. et al. 1990. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell 63: 203–211.
Halenbeck, R., Kawasaki, E., Wrin, J. and Koths, K. 1989. Renaturation and purification of biologically active recombinant human macrophage colony-stimulating factor expressed in E. coli. Bio/Technology 7: 710–715.
Hansson, H.-A., Holmgren, A., Rozell, B. and Stemme, S. 1986. Localization of thioredoxin, thioredoxin reductase and ribonucleotide reductase in cells: immunohistochemical aspects, p. 177–187. In: Thioredoxin and Glutaredoxin Systems: Structure and Function. Holmgren, A., Branden, C.-I., Jornvall, H., and Sjoberg, B.-M.(Eds.). Raven Press, New York.
Maroux, S., Baratti, J. and Desnuelle, P. 1971. Purification and specificity of porcine enterokinase. J. Biol. Chem. 246: 5031–5039.
Paul, S.R., Bennett, F., Calvetti, J.A., Kelleher, K., Wood, C.R., O'Hara, R.M., Leary, A.C., Sibley, B., Clark, S.C., Williams, D.A. and Yang, Y.-C. 1990. Molecular cloning of a cDNA encoding interleukin II, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc. Natl. Acad. Sci. USA 87: 7512–7516.
Scott, J.K. and Smith, G.P. 1990. Searching for peptide ligands with an epitope library. Science 249: 386–390.
Edman, J.C., Ellis, L., Blacher, R.W., Roth, R.A. and Rutter, W.J. 1985. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature 317: 267–270.
Bennett, C.F., Balcarek, J.M., Varrichio, A. and Crooke, S.T. 1988. Molecular cloning and complete amino-acid sequence of form-I phosphoinositide-specific phospholipase C. Nature 334: 268–270.
Mazzarella, R.A., Srinivasan, M., Haugejorden, S.M. and Green, M. 1990. ERp72, an abundant luminal endoplasmic reticulum protein, contains three copies of the active site sequences of protein disulfide isomerase. J. Biol. Chem. 265: 1094–1101.
McCoy, J.M. 1992. Heat-shock proteins and their potential uses for pharmaceutical protein production in microorganisms. In: Stability of Protein Pharmaceuticals, Part B. Ahern, T. and Manning, M. (Eds.) Plenum Press, New York.
Wetzel, R., Perry, L.J., Baase, W.A. and Becktel, W.J. 1988. Disulfide bonds and thermal stability in T4 lysozyme. Proc. Natl. Acad. Sci. USA 85: 401–405.
Schein, C.H. and Noteborn, M.H.M. 1988. Formation of soluble recombinant proteins in Escherichia coli is favored by lower growth temperature. Bio/Technology 6: 291–294.
Joseph-Liauzum, E., Leplatois, P., Legoux, R., Guerverno, V., Marchese, E. and Ferrara, P. 1990. Human recombinant interleukin-1β isolated from Escherichia coli by simple osmotic shock. Gene 86: 291–295.
Rosenwasser, T.A., Hogquist, K.A., Nothwehr, S.F., Bradford-Goldberg, S., Olins, P.O., Chaplin, D.D. and Gordon, J.I. 1990. Compartmentalization of mammalian proteins produced in Escherichia coli. J. Biol. Chem. 265: 13066–13073.
Uhlen, M. and Moks, T. 1990. Gene fusions for purpose of expression: an introduction. Methods in Enzymol. 185: 129–143.
Brent, R. and Ptashne, M. 1981. Mechanism of action of the lex A gene product. Proc. Natl. Acad. Sci. USA 78: 4204–4208.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning, a Laboratory Manual, second edition, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
Norrander, J., Kempe, T. and Messing, J. 1983. Construction of improved M13 vectors using oligonucleotide-directed mutagenesis. Gene 26: 101–106.
Shimatake, H. and Rosenberg, M. 1981. Purified λ regulatory protein cII positively activates promoters for lysogenic development. Nature 292: 128–132.
Woods, S.A., Miles, J.S., Roberts, R.E. and Guest, J.R. 1986. Structural and functional relationships between fumarase and aspartase. Biochem. J. 237: 547–557.
Dorssers, L., Burger, H., Bot, F., Delwel, R., Geurts van Kessel, A., Lowen-berg, B. and Wagemaker, G. 1987. Characterization of a human multilineage-colony-stimulating factor cDNA clone identified by a conserved non-coding sequence in mouse interleukin-3. Gene 55: 115–124.
Zilberstein, A., Ruggieri, R., Korn, J.H. and Revel, M. 1986. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 5: 2529–2537.
Obaru, K., Fukuda, M., Maeda, S. and Shimada, K. 1986. A cDNA clone used to study mRNA inducible in human tonsil lar lymphocytes by a tumor promoter. J. Biochem. 99: 885–894.
Wozney, J.M., Rosen, V., Celeste, A.J., Mitsock, L.M., Whitters, M.J., Kriz, R.W., Hewick, R.M. and Wang, E.A. 1988. Novel regulators of bone formation: molecular clones and activities. Science 242: 1528–1534.
Wong, G.G., Temple, P.A., Leary, A.C., Witek-Giannotti, J.S. et al. 1987 Human CSF-1: molecular cloning and expression of 4-kb cDNA encoding the urinary protein. Science 235: 1504–1508.
Kashima, N., Nishi-Takaoka, C., Fujita, T., Taki, S., Yamada, G., Hamuro, J. and Taniguchi, T. 1985. Unique structure of murine interleukin-2 as deduced from cloned cDNAs. Nature 313: 402–404.
Lee, F., Yokota, T., Otsuka, T., Meyerson, P., Villaret, D., Coffman, R., Mosmann, T., Rennick, D., Roehm, N., Smith, C., Zlotnik, A. and Arai, K.-I. 1986. Isolation and characterization of a mouse interleukin cDNA clone that expressed B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc. Natl. Acad. Sci. USA 83: 2061–2065.
Kinashi, T., Harada, N., Severinson, E., Tanabe, T., Sideras, P., Konishi, M., Azuma, C., Tominaga, A., Bergstedt-Lindqvist, S., Takahashi, M., Matsuda, F., Yaoita, Y., Takatsu, K. and Honjo, T. 1986. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature 324: 70–73.
Gearing, D.P., Gough, N.M., King, J.A., Hilton, D.J., Nicola, N.A., Simpson, R.J., Nice, E.C., Kelso, A. and Metcalf, D. 1987. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J. 6: 3995–4002.
Anderson, D.M., Lyman, S.D., Baird, A., Wignall, J.M., Eisenman, J., Rauch, C., March, C.J., Boswell, H.S., Gimpel, S.D., Cosman, D. and Williams, D.E. 1990. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell 63: 235–243.
Miller, J.H. 1972. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
Micschendahl, M., Petri, T. and Hanggi, U. 1986. A novel prophage independent trp regulated lambda pL expression system. Bio/Technology 4: 802–808.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Schagger, H. and von Jagow, G. 1987. Tricine-sodium dodecyl sulfate-poly-acrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100kDa. Anal. Biochem. 166: 368–379.
Avanzi, C.G., Brizzi, M.F., Giannotti, J., Ciarletta, A., Yang, Y.C., Pegoraro, L. and Clark, S.C. 1990. M-O7e human leukemic factor-dependent cell line provides a rapid and sensitive bioassay for the human cytokines GM-CSF and IL-3. J. Cellular Physiol. 145: 458–464.
Thies, R.S., Bauduy, M., Ashton, B.A., Kurtzberg, L., Wozney, J.M. and Rosen, V. 1992. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology 130: 1318–1324.
Warren, H.S., Hargreaves, J. and Happel, A.J. 1985. Some interleukin-3 dependent mast-cell lines also respond to interleukin-2. Lymphokine Res. 4: 195–204.
Pragnell, I.B., Wright, E.G., Lorimore, S.A., Adam, J., Rosendaal, M., De Lamarter, J.F., Freshney, M., Eckman, L., Sproul, A. and Wilkie, N. 1988. The effect of stem proliferation inhibitors demonstrated with an in vitro assay. Blood 72: 196–199.
Kitamura, T., Tange, T., Chiba, S., Kuwaki, T., Mitani, K., Urabe, A. and Takaku, F. 1989. Establishment and characterization of a unique human cell line that proliferates dependency on GM-CSF, IL-3 or erythropoietin. J. Cellular Physiol. 140: 323–334.
Kawasaki, E.S., Ladner, M.B., Wang, A.M., Van Arsdell, J., Warren, K., Coyne, M.Y., Schweickart, V.L., Lee, M-.T., Wilson, K.J., Boosman, A., Stanley, E.R., Ralph, P. and Mark, D.F. 1986. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-I). Science 230: 291–296.
Moreau, J.-F., Donaldson, D.D., Bennett, F., Witek-Giannotti, J., Clark, S.C. and Wong, G.G. 1988. Leukaemia inhibitory factor is identical to the myeloid growth factor human interleukin for DA cells. Nature 336: 690–692.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LaVallie, E., DiBlasio, E., Kovacic, S. et al. A Thioredoxin Gene Fusion Expression System That Circumvents Inclusion Body Formation in the E. coli Cytoplasm. Nat Biotechnol 11, 187–193 (1993). https://doi.org/10.1038/nbt0293-187
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0293-187
This article is cited by
-
Peptide aptamer targeting Aβ–PrP–Fyn axis reduces Alzheimer’s disease pathologies in 5XFAD transgenic mouse model
Cellular and Molecular Life Sciences (2023)
-
Modeling the role of charged residues in thermophilic proteins by rotamer and dynamic cross correlation analysis
Journal of Molecular Modeling (2023)
-
Retro-protein XXA is a remarkable solubilizing fusion tag for inclusion bodies
Microbial Cell Factories (2022)
-
A toolkit for recombinant production of seven human EGF family growth factors in active conformation
Scientific Reports (2022)
-
Determinants of IGF-II influencing stability, receptor binding and activation
Scientific Reports (2022)