Abstract
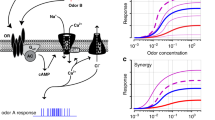
Mammalian urine releases complex mixtures of volatile compounds that are used in reproduction, territoriality and conspecific recognition. To understand how such complex mixtures are represented in the main olfactory bulb, we analysed the electrophysiological responses of individual mitral cells to volatile compounds in mouse urine. In both males and females, urine volatile compounds evoke robust responses in a small subset of mitral cells. Fractionation of the volatile compounds using gas chromatography showed that out of the hundreds of compounds present, mitral cells are activated by single compounds. One cohort of mitral cells responded exclusively to male urine; these neurons were activated by (methylthio)methanethiol, a potent, previously unknown semiochemical present only in male urine. When added to urine, synthetic (methylthio)methanethiol significantly enhances urine attractiveness to female mice. We conclude that mitral cells represent natural odorant stimuli by acting as selective feature detectors, and that their activation is largely independent of the presence of other components in the olfactory stimulus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Baldwin, B. A. & Shillito, E. E. The effects of ablation of the olfactory bulbs on parturition and maternal behaviour in Soay sheep. Anim. Behav. 22, 220–223 (1974)
Schaal, B. et al. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature 424, 68–72 (2003)
Yamaguchi, M. et al. Distinctive urinary odors governed by the major histocompatibility locus of the mouse. Proc. Natl Acad. Sci. USA 78, 5817–5820 (1981)
Brennan, P. A. & Keverne, E. B. Something in the air? New insights into mammalian pheromones. Curr. Biol. 14, R81–R89 (2004)
Dulac, C. & Torello, A. T. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nature Rev. Neurosci. 4, 551–562 (2003)
Andreolini, F., Jemiolo, B. & Novotny, M. V. Dynamics of excretion of urinary chemosignals in the house mouse (Mus musulus) during the natural estrous cycle. Experientia 43, 998–1002 (1987)
Schwende, F. J., Wiesler, D., Jorgenson, J. W., Carmack, M. & Novotny, M. Urinary volatile constituents of the house mouse, Mus musculus, and their endocrine dependency. J Chem. Ecol. 12, 277–295 (1986)
Harvey, S., Jemiolo, B. & Novotny, M. Pattern of volatile compounds in dominant and subordinate male mouse urine. J. Chem. Ecol. 14, 2061–2072 (1989)
Jemiolo, B., Xie, T. M., Andreolini, F., Baker, A. E. M. & Novotny, M. The t complex of the mouse: chemical characterization by urinary volatile profiles. J. Chem. Ecol. 17, 353–367 (1990)
Schaefer, M. L., Young, D. A. & Restrepo, D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J. Neurosci. 21, 2481–2487 (2001)
Schaefer, M. L., Yamazaki, K., Osada, K., Restrepo, D. & Beauchamp, G. K. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J. Neurosci. 22, 9513–9521 (2002)
Lodovichi, C., Belluscio, L. & Katz, L. C. Functional topography of connections linking mirror-symmetric maps in the mouse olfactory bulb. Neuron 38, 265–276 (2003)
Belluscio, L., Lodovichi, C., Feinstein, P., Mombaerts, P. & Katz, L. C. Odorant receptors instruct functional circuitry in the mouse olfactory bulb. Nature 419, 296–300 (2002)
Wadhams, L. Coupled gas chromatography-single cell recording: a new technique for use in the analysis of insect pheromones. Z. Naturforsch. 37c, 947–952 (1982)
Stensmyr, M. C., Giordano, E., Balloi, A., Angioy, A. M. & Hansson, B. S. Novel natural ligands for Drosophila olfactory receptor neurones. J. Exp. Biol. 206, 715–724 (2003)
Kayali-Sayadi, M. N., Bautista, J. M., Polo-Diez, L. M. & Salazar, I. Identification of pheromones in mouse urine by head-space solid phase microextraction followed by gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 796, 55–62 (2003)
Louch, D., Motlagh, S. & Pawliszyn, J. Dynamics of organic compound extraction from water using liquid-coated fused silica fibers. Anal. Chem. 64, 1187–1199 (1992)
Baek, H. H. & Kim, H. J. Solid phase microextraction-gas chromatography-olfactometry of soy sauce based on sample dilution analysis. Food Sci. Biotech. 13, 90–95 (2004)
Lee, S. N., Kim, N. S. & Lee, D. S. Comparative study of extraction techniques for determination of garlic flavor components by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 337, 749–756 (2003)
Torrens, J., Rui-Aumatell, M., Lopez-Tamames, E. & Buxaderas, S. Volatile compounds of red and white wines by headspace ETH solid-phase microextraction using different fibers. J. Chromatogr. Sci. 42, 310–316 (2004)
Novotny, M. V. et al. A unique urinary constituent, 6-hydroxy-6-methyl-3-heptanone, is a pheromone that accelerates puberty in female mice. Chem. Biol. 6, 377–383 (1999)
Schutte, L. One-step synthesis of dithiohemiacetals, a new class of compounds. Tetrahedr. Lett. 12, 2321–2322 (1971)
Singer, A. G. et al. Dimethyl disulfide: an attractant pheromone in hamster vaginal secretion. Science 191, 948–950 (1976)
Galef, B. G. Jr, Mason, J. R., Preti, G. & Bean, N. J. Carbon disulfide: a semiochemical mediating socially-induced diet choice in rats. Physiol. Behav. 42, 119–124 (1988)
Scott, J. W. & Pfaff, D. W. Behavioral and electrophysiological responses of female mice to male urine odors. Physiol. Behav. 5, 407–411 (1970)
Doty, R. L., Green, P. A., Ram, C. & Yankell, S. L. Communication of gender from human breath odors: relationship to perceived intensity and pleasantness. Horm. Behav. 16, 13–22 (1982)
Wallace, P. Individual discrimination of humans by odor. Physiol. Behav. 19, 577–579 (1977)
Novotny, M., Schwende, F. J., Wiesler, D., Jorgenson, J. W. & Carmack, M. Identification of a testosterone-dependent unique volatile constituent of male mouse urine: 7-exo-ethyl-5-methyl-6,8-dioxabicyclo[3.2.1]-3-octene. Experientia 40, 217–219 (1984)
Leinders-Zufall, T. et al. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature 405, 792–796 (2000)
Novotny, M., Harvey, S. & Jemiolo, B. Chemistry of male dominance in the house mouse, Mus domesticus . Experientia 46, 109–113 (1990)
Shinoda, K., Shiotani, Y. & Osawa, Y. “Necklace olfactory glomeruli” form unique components of the rat primary olfactory system. J. Comp. Neurol. 284, 362–373 (1989)
Weruaga, E. et al. A sexually dimorphic group of atypical glomeruli in the mouse olfactory bulb. Chem. Senses 26, 7–15 (2001)
Lin, W., Arellano, J., Slotnick, B. & Restrepo, D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J. Neurosci. 24, 3703–3710 (2004)
Hildebrand, J. G. & Shepherd, G. M. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu. Rev. Neurosci. 20, 595–631 (1997)
Mori, K. Relation of chemical structure to specificity of response in olfactory glomeruli. Curr. Opin. Neurobiol. 5, 467–474 (1995)
Friedrich, R. W. & Laurent, G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science 291, 889–894 (2001)
Motokizawa, F. Odor representation and discrimination in mitral/tufted cells of the rat olfactory bulb. Exp. Brain Res. 112, 24–34 (1996)
Tanabe, T., Iino, M. & Takagi, S. F. Discrimination of odors in olfactory bulb, pyriform-amygdaloid areas, and orbitofrontal cortex of the monkey. J. Neurophysiol. 38, 1284–1296 (1975)
Friedrich, R. W., Habermann, C. J. & Laurent, G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nature Neurosci. 7, 862–871 (2004)
Wang, J. W., Wong, A. M., Flores, J., Vosshall, L. B. & Axel, R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271–282 (2003)
Wilson, R. I., Turner, G. C. & Laurent, G. Transformation of olfactory representations in the Drosophila antennal lobe. Science 303, 366–370 (2004)
Vosshall, L. B., Amrein, H., Morozov, P. S., Rzhetsky, A. & Axel, R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 96, 725–736 (1999)
Clyne, P. J. et al. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila . Neuron 22, 327–338 (1999)
Ngai, J., Dowling, M. M., Buck, L., Axel, R. & Chess, A. The family of genes encoding odorant receptors in the channel catfish. Cell 72, 657–666 (1993)
Miyashita, K. & Robinson, A. B. Identification of compounds in mouse urine vapor by gas chromatography and mass spectrometry. Mech. Ageing Dev. 13, 177–184 (1980)
Bocchini, P., Andalo, C., Bonfiglioli, D. & Galletti, G. C. Solid-phase microextraction gas chromatography/mass spectrometric analysis of volatile organic compounds in water. Rapid Commun. Mass Spectrom. 13, 2133–2139 (1999)
Contarini, G. & Povolo, M. Volatile fraction of milk: comparison between purge and trap and solid phase microextraction techniques. J. Agric. Food Chem. 50, 7350–7355 (2002)
Fu, S. G., Yoon, Y. & Bazemore, R. Aroma-active components in fermented bamboo shoots. J. Agric. Food Chem. 50, 549–554 (2002)
Brody, C. D. & Hopfield, J. J. Simple networks for spike-timing-based computation, with application to olfactory processing. Neuron 37, 843–852 (2003)
Laurent, G. Olfactory network dynamics and the coding of multidimensional signals. Nature Rev. Neurosci. 3, 884–895 (2002)
Acknowledgements
We thank R. Axel, M. Ehlers, R. Mooney, D. Fitzpatrick and members of the Katz laboratory for critical comments on the manuscript; J. Jin, R. Irving, G. Dubay and L. Nielsen for technical assistance; and X. Han for critical assistance with chemical analysis. This work is supported by the NIH (NICDS) (L.C.K.), the Broad Foundation (D.Y.L.), NSF (E.B.), the Petroleum Research Fund, administered by the American Chemical Society (E.B.), and the Berryman Institute (E.B.). L.C.K. is an Investigator in the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Methods
This file contains details on constructing cell response map and synthesis of (methylthio)methanethiol. (DOC 28 kb)
Supplementary Figure S1
Clustered organization of urine responsive neurons in the main olfactory bulb. (PPT 263 kb)
Supplementary Figure S2
Optical imaging of intrinsic signals reveals no glomerular activation in response to urine odour on dorsal surface of the MOB. (PPT 4482 kb)
Supplementary Figure S3
Schematic representation of a gas chromatography-electrophysiology (GC-E) experiment. (PPT 80 kb)
Supplementary Figure S4
Urine responsive cells activated by multiple compounds in urine. (PPT 340 kb)
Supplementary Figure S5
Averaging multiple GC runs does not reveal the presence of additional weak responses. (PPT 608 kb)
Supplementary Figure S6
Separated compounds present in urine provide a rich source of odourant stimuli detectable by human observers. (PPT 94 kb)
Supplementary Figure S7
The pheromone 6-hydoxy-6-methyl-3-heptanone (6H6M3H) is not responsible for excitation of male-urine selective mitral cells. (PPT 195 kb)
Supplementary Figure S8
Scatter plots comparing the investigation times for intact, castrated and MTMT-containing urine. (PPT 58 kb)
Supplementary Figure S9
Neuronal responses elicited by MTMT are independent of other components in castrated mouse urine. (PPT 559 kb)
Supplementary Figure Legends
Legends to accompany the above Supplementary Figures S1-S9. (DOC 36 kb)
Rights and permissions
About this article
Cite this article
Lin, D., Zhang, SZ., Block, E. et al. Encoding social signals in the mouse main olfactory bulb. Nature 434, 470–477 (2005). https://doi.org/10.1038/nature03414
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature03414
This article is cited by
-
Social odor choice buffers drug craving
Neuropsychopharmacology (2024)
-
Sulfur-Markers, From Urine of Fishing Cat: the Putative Pheromonal Compounds of Water-Loving, Vulnerable State Animal of West Bengal
Proceedings of the Zoological Society (2023)
-
Hemoglobin in the blood acts as a chemosensory signal via the mouse vomeronasal system
Nature Communications (2022)
-
Amylin-Calcitonin receptor signaling in the medial preoptic area mediates affiliative social behaviors in female mice
Nature Communications (2022)
-
Neural circuit control of innate behaviors
Science China Life Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.