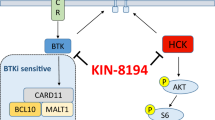
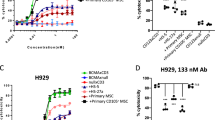
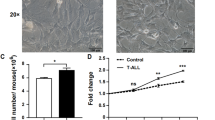
Abstract
This study explores whether lymphoma cell adhesion-induced B cell-activating factor (BAFF) expression in bone marrow stromal cells (BMSCs) protects B lymphoma cells from apoptosis. We first showed protection of lymphoma cells from apoptosis by conditioned medium of a stromal cell-lymphoma cell coculture, either spontaneous or induced by mitoxantrone, implying a role for soluble factor(s) in lymphoma cell survival. Addition of BAFF counteracted mitoxantrone-induced apoptosis and elicited a reduction in spontaneous apoptosis in primary lymphomas, suggesting a role of BAFF in sustaining B-cell survival. Abundant BAFF was detected in the BMSC cell line (HS-5) and primary BMSCs by flow cytometry, RT-PCR and immunoblotting. BAFF levels were 20- to 200-fold higher in BMSCs than in lymphoma cells, and lymphoma cell adhesion to BMSCs augmented BAFF secretion twofold through upregulation of BAFF gene expression. Finally, neutralization of BAFF by TACI-Ig or depletion of BAFF by small hairpin RNA (shRNA) in BMSCs significantly enhanced lymphoma cell response to chemotherapy and overcame stroma-mediated drug resistance, suggesting that lymphoma cells use BMSC-derived BAFF as a survival factor. These findings support the hypothesis that lymphoma cells interact with BMSCs, resulting in stromal niches with high BAFF concentration, and identify BMSC-derived BAFF as a functional determinant for B lymphoma cell survival in the bone marrow environment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Meads MB, Hazlehurst LA, Dalton WS . The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res 2008; 14: 2519–2526.
Shu HB, Hu WH, Johnson H . TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol 1999; 65: 680–683.
Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S . Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med 2000; 192: 953–964.
Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 2000; 404: 995–999.
Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 2001; 293: 2111–2114.
Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 1999; 190: 1697–1710.
Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci USA 2000; 97: 3370–3375.
Grumont RJ, Rourke IJ, Gerondakis S . Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev 1999; 13: 400–411.
Mercurio F, DiDonato JA, Rosette C, Karin M . p105 and p98 precursor proteins play an active role in NF-κB-mediated signal transduction. Genes Dev 1993; 7: 705–718.
Naumann M, Wulczyn FG, Scheidereit C . The NF-κB precursor p105 and the proto-oncogene product Bcl-3 are IκB molecules and control nuclear translocation of NF-κB. EMBO J 1993; 12: 213–222.
Liou HC, Nolan GP, Ghosh S, Fujita T, Baltimore D . The NF-κB p50 precursor, p105, contains an internal IκB-like inhibitor that preferentially inhibits p50. EMBO J 1992; 11: 3003–3009.
Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG et al. BAFF-R, a newly identified TNF receptor that specifically interact with BAFF. Science 2001; 293: 2108–2111.
Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M et al. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol 2000; 1: 252–256.
Marsters SA, Yan M, Pitti RM, Haas PE, Dixit VM, Askenazi A . Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol 2000; 10: 785–788.
Shu HB, Johnson H . B cell maturation protein is a receptor for the tumor necrosis factor family member TALL-1. Proc Natl Acad Sci USA 2000; 97: 9156–9161.
Briones J, Timmerman JM, Hilbert DM, Levy R . BLyS and BLyS receptor expression in non-Hodgkin's lymphoma. Exp Hematol 2002; 30: 135–141.
Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood 2004; 103: 689–694.
Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood 2004; 103: 679–688.
Moreaux J, Legouffe E, Jourdan E, Quittet P, Rème T, Lugagne C et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004; 103: 3148–3157.
Lwin T, Hazlehurst LA, Moscinski L, Dalton WS, Tao J . Bone marrow stroma (BMS) prevents apoptosis of lymphoma cells by upregulation of BMS-derived B-cell activating factor (BAFF) and activation of NF-κB. Leukemia 2007; 21: 1521–1531.
Nefedova Y, Landowski TH, Dalton WS . Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia 2003; 17: 1175–1182.
Lwin T, Hazlehurst LA, Sotomayor E, Moscinski LC, Dalton WS, Tao J . Cell adhesion induces p27Kip1-associated cell-cycle arrest through down-regulating SCFSkp2 ubiquitin ligase pathway in mantle cell and other non-Hodgkin's B-cell lymphomas. Blood 2007; 110: 1631–1638.
Elsawa SF, Novak AJ, Grote DM, Ziesmer SC, Witzig TE, Kyle RA et al. B-lymphocyte stimulator (BLyS) stimulates immunoglobulin production and malignant B-cell growth in Waldenstrom's macroglobulinemia. Blood 2005; 107: 2882–2888.
Jelinek DF, Darce JR . Human B lymphocyte malignancies: exploitation of BLyS and APRIL and their receptors. Curr Dir Autoimmun 2005; 8: 266–288.
Novak AJ, Bram RJ, Kay NE, Jelinek DF . Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood 2002; 100: 2973–2979.
Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res 2006; 66: 6675–6682.
Fu L, Lin-Lee YC, Pham LV, Tamayo A, Yoshimura L, Ford RJ . Constitutive NF-kappaB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas. Blood 2006; 107: 4540–4548.
Acknowledgements
This work was supported by grants from the American Cancer Society IRG 032 ACS (JT),the National Cancer Institute and the National Institute on Aging, and by a Cancer Pilot Research Grant (JT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lwin, T., Crespo, L., Wu, A. et al. Lymphoma cell adhesion-induced expression of B cell-activating factor of the TNF family in bone marrow stromal cells protects non-Hodgkin's B lymphoma cells from apoptosis. Leukemia 23, 170–177 (2009). https://doi.org/10.1038/leu.2008.266
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2008.266
Keywords
This article is cited by
-
Treatment resistance in diffuse large B-cell lymphoma
Leukemia (2021)
-
APRIL and BAFF: novel biomarkers for central nervous system lymphoma
Journal of Hematology & Oncology (2019)
-
Unification of de novo and acquired ibrutinib resistance in mantle cell lymphoma
Nature Communications (2017)
-
Bone marrow stromal cells induced activation of nuclear factor κB signaling protects non-Hodgkin’s B lymphoma cells from apoptosis
Tumor Biology (2016)
-
The tumor microenvironment shapes hallmarks of mature B-cell malignancies
Oncogene (2015)