Abstract
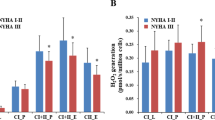
We investigated whether circulating leucocytes from hypertensive patients exhibit more spontaneous, stimulated hydrogen peroxide (H2O2) production and greater mitochondrial membrane potential (Δψ) than those from normotensive individuals. We also investigated the effects of oral treatment with the angiotensin II (AT II) type 1 receptor blocker eprosartan (600 mg day−1) on these markers of oxidative stress. In 25 hypertensive patients and 28 healthy volunteers, spontaneous H2O2 formation was measured by flow cytometry after preincubation of buffy coat-leucocytes from fresh peripheral venous blood at 37 °C with 2′,7′ dichlorofluorescein. Stimulation of H2O2 formation by circulating leucocytes was elicited by the addition of tert-butylhydroperoxide (tBHP). Δψ was determined by flow cytometry after the addition of tetramethylrhodamine methyl ester (TMRM). Compared with healthy individuals, lymphocytes from hypertensive patients exhibited higher Δψ (12.28±3.20 vs 16.25±2.88 arbitrary fluorescence units (AFU), respectively; P<0.001) and greater spontaneous H2O2 production (4.75±5.15 vs 8.98±9.97 AFU, respectively; P<0.05). tBHP stimulation was associated with higher H2O2 levels in circulating leucocytes in patients with uncorrected hypertension than in normotensive individuals. H2O2 overproduction was corrected by eprosartan treatment. These results suggest that oxidative stress could be important in the pathogenesis of hypertension. Furthermore, measurement of leucocyte oxidant activities may be useful for the evaluation of oxidative stress, which may be reduced with the use of antihypertensive drugs. Our results demonstrate that treatment of hypertension with eprosartan normalizes blood pressure and corrects oxidative disturbances, suggesting that leucocytes could be a target for this drug.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kim CH, Vaziri ND . Hypertension promotes integrin expression and reactive oxygen species generation by circulating leucocytes. Kidney Int 2005; 67: 1462–1470.
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K . Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med 2002; 346: 1954–1962.
Shen K, DeLano FA, Zweifach BW, Schmid-Schonbein GW . Circulating leucocytes counts, activation, and degranulation in Dahl hypertensive rats. Circ Res 1995; 76: 276–283.
Dorffel Y, Latsch C, Stuilmuller B, Schreiber S, Scholze S, Burmester GR et al. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension 1999; 34: 113–117.
Yasunari K, Maeda K, Nakamura M, Yoshikawa J . Oxidative stress in leucocytes is a possible link between blood pressure, blood glucose, and C-reactive protein. Hypertension 2002; 39: 777–780.
Cadenas E, Davies KJ . Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 2000; 29: 222–230.
Boveris A, Chance B . The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 1973; 134: 707–716.
Salvador A, Sousa J, Pinto RE . Hydroperoxyl, superoxide and pH gradients in the mitochondrial matrix: a theoretical assessment. Free Radic Biol Med 2001; 1: 1208–1215.
Nulton-Persson AC, Szweda LI . Modulation of mitochondrial function by hydrogen peroxide. J Biol Chem 2001; 276: 23357–23361.
Kowaltowski AJ, Vercesi AH . Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med 1999; 26: 463–471.
Teveten (Eprosartan) Summary of Product Characteristics. Solvay Healthcare Limited: Southampton, UK, 2006.
Manabe S, Takafumi O, Watanabe S, Fukuoka T, Jitsuo H . Effects of angiotensin II receptor blockade with valsartan on proinflammatory cytokines in patients with essential hypertension. J Cardiovasc Pharmacol 2005; 46: 735–739.
Huang SG . Development of a high throughput screening assay for mitochondrial membrane potential in living cells. J Biomol Screen 2002; 7: 383–389.
Scaduto RC, Grotyohann LW . Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 1999; 76: 469–477.
LeBel CP, Ischiropoulos H, Bondy SC . Evaluation of the probe 2′,7′-dichlorofluorescein as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 1992; 5: 227–231.
Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M . Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol 1983; 130: 1910–1917.
Vaziri ND, Rodríguez-Iturbe B . Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2006; 2: 582–593.
Halliwell B . Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med 1999; 91 (Suppl 3C): 14S–22S.
Crakowki JL, Baguet JP, Ormezzano O, Bessard J, Stanke-Labesque F, Bessard G et al. Lipid peroxidation is not increased in patients with untreated mild-to-moderate hypertension. Hypertension 2003; 41: 286–288.
Abeles FB . Plant chemiluminescence. Ann Rev Plant Physiol 1986; 37: 49–72.
Lacy F, O'Connor DT, Schmid-Schonbein GW . Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J Hypertens 1998; 16: 291–303.
Karnovsky ML . The metabolism of leucocytes. Semin Hematol 1968; 5: 156–159.
Fossati G, Moulding DA, Spiller DG, Moots RJ, White MRH, Edwards SW . The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J Immunol 2003; 170: 1964–1972.
El Bekay R, Alvarez M, Monteseirin J, Alba G, Chacón P, Vega A et al. Oxidative stress is a critical mediator of the angiotensin II signal in human neutrophils: involvement of mitogen-activated protein kinase, calcineurin, and the transcription factor NK-κB. Blood 2003; 102: 662–671.
Schmeisser A, Soehnlein O, Illmer T, Lorenz HM, Eskafi S, Roerick O et al. ACE inhibition lowers angiotensin II-induced chemokine expression by reduction of NF-kappa B activity and AT1 receptor expression. Biochem Biophys Res Commun 2004; 352: 532–540.
Kim JA, Berliner JA, Nadler JR . Angiotensin II increases monocyte binding to endothelial cells. Biochem Biophys Res Commun 1996; 226: 862–868.
Labiós M, Martínez M, Gabriel F, Guiral V, Muñoz A, Aznar J . Effect of eprosartan on cytoplasmic free calcium mobilization, platelet activation, and microparticle formation in hypertension. Am J Hypertens 2004; 17: 757–763.
Li N, Hu H, Lindqvist M, Wikström-Jonsson E, Goodall AH, Hjemdahl P . Platelet–leucocyte cross talk in whole blood. Arterioscler Thromb Vasc Biol 2000; 20: 2702–7208.
Bazzoni G, Dejana E, Del Maschio A . Platelet–neutrophil interactions. Possible relevance in the pathogenesis of thrombosis and inflammation. Haematologica 1991; 76: 491–499.
Lijnen P, Fagard R, Petrov V . Cytosolic calcium changes induced by angiotensin II in human peripheral blood mononuclear cells are mediated via angiotensin II subtype 1 receptors. J Hypertens 1997; 15: 871–876.
Sela S, Shurtz-Swirski R, Farah R, Levy R, Shapiro G, Chezar J et al. A link between polymorphonuclear leucocyte intracellular calcium, plasma insulin, and essential hypertension. Am J Hypertens 2002; 15: 291–295.
Simic DV, Mimik-Oka J, Pljesa-Ercegovac M, Savic-Radojevic A, Opacic M, Matic D et al. Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma of patients with different degrees of essential hypertension. J Hum Hypertens 2006; 20: 149–155.
Germanó S, Sanguigni V, Pignatelli P, Caccese D, Lenti L, Ragazzo M et al. Enhanced platelet release of superoxide anion in systemic hypertension: role of AT1 receptors. J Hypertens 2004; 22: 1085–1086.
Zhang H, Schmeisser A, Garlichs CD, Plotze K, Damme U, Mugge A et al. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc Res 1999; 44: 215–222.
Acknowledgements
This study was supported by funding from Solvay Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Labiós, M., Martínez, M., Gabriel, F. et al. Effects of eprosartan on mitochondrial membrane potential and H2O2 levels in leucocytes in hypertension. J Hum Hypertens 22, 493–500 (2008). https://doi.org/10.1038/jhh.2008.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2008.19
Keywords
This article is cited by
-
Protective and therapeutic role of mango pulp and eprosartan drug and their anti-synergistic effects against thioacetamide-induced hepatotoxicity in male rats
Environmental Science and Pollution Research (2022)
-
A novel bifunctional mitochondria-targeted anticancer agent with high selectivity for cancer cells
Scientific Reports (2015)