Abstract
Rationale:
Fatty acid amide hydrolase (FAAH) is the main degrading enzyme of the fatty acid ethanolamides anandamide (AEA) and oleoylethanolamide (OEA), which have opposite effects on food intake and energy balance. AEA, an endogenous ligand of CB1 cannabinoid receptors, enhances food intake and energy storage, whereas OEA binds to peroxisome proliferator-activated receptors-α to reduce food intake and promoting lipolysis. To elucidate the role of FAAH in food intake and energy balance, we have evaluated different metabolic and behavioral responses related to feeding in FAAH-deficient (FAAH−/−) mice and their wild-type littermates.
Methodology and Results:
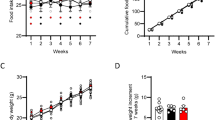
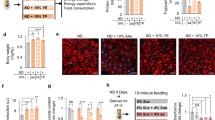
Total daily food intake was similar in both genotypes, but high-fat food consumption was enhanced during the dark hours and decreased during the light hours in FAAH−/− mice. The reinforcing and motivational effects of food were also enhanced in FAAH−/− mice as revealed by operant behavioral paradigms. These behavioral responses were reversed by the administration of the selective CB1 cannabinoid antagonist rimonabant. Furthermore, body weight, total amount of adipose tissue, plasma-free fatty acids and triglyceride content in plasma, liver, skeletal muscle and adipose tissue, were increased in FAAH−/− mice. Accordingly, leptin levels were increased and adiponectin levels decreased in these mutants, FAAH−/− mice also showed enhanced plasma insulin and blood glucose levels revealing an insulin resistance. As expected, both AEA and OEA levels were increased in hypothalamus, small intestine and liver of FAAH−/− mice.
Conclusion:
These results indicate that the lack of FAAH predominantly promotes energy storage by food intake-independent mechanisms, through the enhancement of AEA levels rather than promoting the anorexic effects of OEA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Cota D . CB1 receptors: emerging evidence for central and peripheral mechanisms that regulate energy balance, metabolism, and cardiovascular health. Diabetes Metab Res Rev 2007; 23: 507–517.
Colombo G, Agabio R, Díaz G, Lobina C, Reali R, Gessa GL . Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci 1998; 63: L113–L117.
Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S . Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005; 365: 1389–1397.
Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 2003; 112: 423–431.
Jamshidi N, Taylor DA . Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 200; 134: 1151–1154.
Kirkham TC, Williams CM, Fezza F, Di Marzo V . Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol 2002; 136: 550–557.
Thornton-Jones ZD, Vickers SP, Clifton PG . The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology (Berl) 2005; 179: 452–460.
Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ . Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci 2004; 24: 2708–2715.
Pertwee RG . Cannabinoids and the gastrointestinal tract. Gut 2001; 48: 859–867.
Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le FG, Oury-Donat F et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 2003; 63: 908–914.
Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 2005; 115: 1298–1305.
Williams CM, Kirkham TC . Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999; 143: 315–317.
Mahler SV, Smith KS, Berridge KC . Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology 2007; 32: 2267–2278.
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB . Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996; 384: 83–87.
Ueda N, Yamamoto S . Anandamide amidohydrolase (fatty acid amide hydrolase). Prostaglandins Other Lipid Mediat 2000; 61: 19–28.
Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K et al. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem 2007; 282: 1518–1528.
Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D . Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci 2005; 62: 708–716.
Guzman M, Lo VJ, Fu J, Oveisi F, Blazquez C, Piomelli D . Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha). J Biol Chem 2004; 279: 27849–27854.
Kunos G . Understanding metabolic homeostasis and imbalance: what is the role of the endocannabinoid system? Am J Med 2007; 120 (Suppl 1): S18–S24.
Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA 2001; 98: 9371–9376.
Gaetani S, Oveisi F, Piomelli D . Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology 2003; 28: 1311–1316.
Giuffrida A, Rodríguez de Fonseca F, Nava F, Loubet-Lescoulie P, Piomelli D . Elevated circulating levels of anandamide after administration of the transport inhibitor, AM404. Eur J Pharmacol 2000; 408: 161–168.
Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 2005; 313: 352–358.
Cota D . Role of the endocannabinoid system in energy balance regulation and obesity. Front Horm Res 2008; 36: 135–145.
Fu J, Gaetani S, Oveisi F, Lo VJ, Serrano A, Rodríguez de Fonseca F et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 2003; 425: 90–93.
Rodríguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J et al. An anorexic lipid mediator regulated by feeding. Nature 2001; 414: 209–212.
Matias I, Bisogno T, Di Marzo V . Endogenous cannabinoids in the brain and peripheral tissues: regulation of their levels and control of food intake. Int J Obes (Lond) 2006; 30 (Suppl 1): S7–S12.
Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007; 6: 414–421.
Valenti M, Vigano D, Casico MG, Rubino T, Steardo L, Parolaro D et al. Differential diurnal variations of anandamide and 2-arachidonoyl-glycerol levels in rat brain. Cell Mol Life Sci 2004; 61: 945–950.
Gamber KM, Macarthur H, Westfall TC . Cannabinoids augment the release of neuropeptide Y in the rat hypothalamus. Neuropharmacology 2005; 49: 646–652.
Huang H, cuna-Goycolea C, Li Y, Cheng HM, Obrietan K, van den Pol AN . Cannabinoids excite hypothalamic melanin-concentrating hormone but inhibit hypocretin/orexin neurons: implications for cannabinoid actions on food intake and cognitive arousal. J Neurosci 2007; 27: 4870–4881.
Osei-Hyiaman D, DePetrillo M, Harvey-White J, Bannon AW, Cravatt BF, Kuhar MJ et al. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology 2005; 81: 273–282.
Ravinet TC, Delgorge C, Menet C, Arnone M, Soubrie P . CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 2004; 28: 640–648.
Lefebvre P, Chinetti G, Fruchart JC, Staels B . Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 2006; 116: 571–580.
Matias I, Gonthier MP, Petrosino S, Docimo L, Capasso R, Hoareau L et al. Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells. Br J Pharmacol 2007; 152: 676–690.
Ward SJ, Dykstra LA . The role of CB1 receptors in sweet versus fat reinforcement: effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940). Behav Pharmacol 2005; 16: 381–388.
Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF . Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience 2006; 137: 337–361.
Fu J, Kim J, Oveisi F, Astarita G, Piomelli D . Targeted enhancement of oleoylethanolamide production in proximal small intestine induces across-meal satiety in rats. Am J Physiol Regul Integr Comp Physiol 2008; 295: R45–R50.
Motter AL, Ahern GP . TRPV1-null mice are protected from diet-induced obesity. FEBS Lett 2008; 582: 2257–2262.
Acknowledgements
This study was supported by grants from European Communities (GENADDICT LSHM-CT-2004-005166 and PHECOMP LHSM-CT-2006-037669), National Institute on Drug Abuse (NIDA) (DA012413), Instituto de Salud Carlos III (RD06/001/001), Spanish Ministry of Education (SAF2007-64062) and Generalitat de Catalunya (2005SGR00131). CT was financed by FI and BE fellowships from AGAUR (Generalitat de Catalunya).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Touriño, C., Oveisi, F., Lockney, J. et al. FAAH deficiency promotes energy storage and enhances the motivation for food. Int J Obes 34, 557–568 (2010). https://doi.org/10.1038/ijo.2009.262
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.262
Keywords
This article is cited by
-
Association between the FAAH C385A variant (rs324420) and obesity-related traits: a systematic review
International Journal of Obesity (2024)
-
Inhibition of fatty acid binding protein-5 in the basolateral amygdala induces anxiolytic effects and accelerates fear memory extinction
Psychopharmacology (2024)
-
The CB1 cannabinoid receptor regulates autophagy in the tibialis anterior skeletal muscle in mice
Biological Research (2023)
-
Lower amygdala fatty acid amide hydrolase in violent offenders with antisocial personality disorder: an [11C]CURB positron emission tomography study
Translational Psychiatry (2021)
-
High-fat but not normal-fat intake of extra virgin olive oil modulates the liver proteome of mice
European Journal of Nutrition (2021)