Abstract
Introduction:
We utilized a genomic analysis of the response of neuronal GT1-7 cells to enterostatin to identify pathways responsive to this peptide. This information, together with reported properties of the enterostatin receptor, suggested that enterostatin may have an effect on angiogenesis.
Method:
To investigate this hypothesis, we studied the effect of enterostatin as an antiangiogenic agent in two angiogenic tissue culture model systems.
Results:
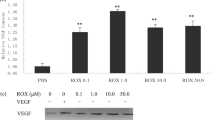
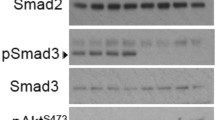
Enterostatin induced a 50% or greater inhibition in the angiogenic response of human fat cells and had a U-shaped bimodal dose–response effect in inhibiting angiogenesis in a human placental vein angiogenesis model. To further understand this response, we tested enterostatin's effect in a human hepatoma cell line (HepG2 cells) that was subjected to glucose deprivation, a condition known to induce angiogenesis in other tumor cell lines. Phosphorylated AMP kinase (pAMPK) levels and vascular endothelial growth factor A (VEGF-A) mRNA expression were elevated robustly after incubation of HepG2 cells in the absence of glucose for 4 h, but 15 min incubation with enterostatin dramatically inhibited this pAMPK activation and reduced VEGF-A gene expression after 1 h incubation with enterostatin. The AMPK activator 5-aminoimidazole-4-carboximide ribonucleoside (AICAR) induced VEGF-A expression.
Summary:
These data suggest that enterostatin has an antiangiogenic effect and suggest that it regulates VEGF-A gene expression through inhibition of AMPK activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lin L, Park M, York DA . Enterostatin inhibition of dietary fat intake is modulated through the melanocortin system. Peptides 2007; 28: 643–649.
York DA, Park MJ . Enterostatin, a peptide regulator of dietary fat ingestion. In: Kastan A (ed). Biologically Active Peptides. Elsevier Inc.: San Diego, CA, 2006, pp 969–974.
Okada S, York DA, Bray GA, Mei J, Erlanson-Albertsson C . Differential inhibition of fat intake in two strains of rat by the peptide enterostatin. Am J Physiol 1992; 262: R1111–R1116.
Okada S, York DA, Bray GA . Procolipase mRNA: tissue localization and effects of diet and adrenalectomy. Biochem J 1993; 292 (Part 3): 787–789.
Sorhede M, Mulder H, Mei J, Sundler F, Erlanson-Albertsson C . Procolipase is produced in the rat stomach—a novel source of enterostatin. Biochim Biophys Acta 1996; 1301: 207–212.
Lin L, Park M, Hulver M, York DA . Different metabolic responses to central and peripheral injection of enterostatin. Am J Physiol Regul Integr Comp Physiol 2006; 290: R909–R915.
Ookuma M, York DA . Inhibition of insulin release by enterostatin. Int J Obes Relat Metab Disord 1998; 22: 800–805.
Park M, Lin L, Thomas S, Braymer HD, Smith PM, Harrison DH et al. The F1-ATPase beta-subunit is the putative enterostatin receptor. Peptides 2004; 25: 2127–2133.
Berger K, Sivars U, Winzell MS, Johansson P, Hellman U, Rippe C et al. ATP synthase—a direct target in appetite regulation and energy balance. Nutr Neurosci 2002; 5: 201–210.
Berger K, Sivars U, Winzell MS, Johansson P, Hellman U, Rippe C et al. Mitochondrial ATP synthase—a possible target protein in the regulation of energy metabolism in vitro and in vivo. Nutr Neurosci 2002; 5: 201–210.
Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezón E, Champagne E et al. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature 2003; 421: 75–79.
Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK et al. Endothelial cell surface F1–F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci USA 2001; 98: 6656–6661.
Moser TL, Stack MS, Asplin I, Enghild JJ, Højrup P, Everitt L et al. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA 1999; 96: 2811–2816.
Kim BW, Choo HJ, Lee JW, Kim JH, Ko YG . Extracellular ATP is generated by ATP synthase complex in adipocyte lipid rafts. Exp Mol Med 2004; 36: 476–485.
Das B, Mondragon MO, Sadeghian M, Hatcher VB, Norin AJ . A novel ligand in lymphocyte-mediated cytotoxicity: expression of the beta subunit of H+ transporting ATP synthase on the surface of tumor cell lines. J Exp Med 1994; 180: 273–281.
Neurath KM, Keough MP, Mikkelsen T, Claffey KP . AMP-dependent protein kinase alpha 2 isoform promotes hypoxia-induced VEGF expression in human glioblastoma. Glia 2006; 53: 733–743.
Ouchi N, Shibata R, Walsh K . AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res 2005; 96: 838–846.
Greenway FL, Liu Z, Yu Y, Caruso MK, Roberts AT, Lyons J et al. An assay to measure angiogenesis in human fat tissue. Obes Surg 2007; 17: 510–515.
Woltering EA, Lewis JM, Maxwell IV PJ, Frey DJ, Wang YZ, Rothermel J et al. Development of a novel in vitro human tissue-based angiogenesis assay to evaluate the effect of antiangiogenic drugs. Ann Surg 2003; 237: 790–798; discussion 798–800.
Hornick CA, Myers A, Sadowska-Krowicka H, Anthony CT, Woltering EA . Inhibition of angiogenic initiation and disruption of newly established human vascular networks by juice from Morinda citrifolia (noni). Angiogenesis 2003; 6: 143–149.
Brown KJ, Maynes SF, Bezos A, Maguire DJ, Ford MD, Parish CR et al. A novel in vitro assay for human angiogenesis. Lab Invest 1996; 75: 539–555.
Yun H, Lee M, Kim SS, Ha J . Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma. J Biol Chem 2005; 280: 9963–9972.
Champagne E, Martinez LO, Collet X, Barbaras R . Ecto-F1Fo ATP synthase/F1 ATPase: metabolic and immunological functions. Curr Opin Lipidol 2006; 17: 279–284.
Lin L, Okada S, York DA, Bray GA . Structural requirements for the biological activity of enterostatin. Peptides 1994; 15: 849–854.
Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 2007; 56: 1517–1526.
Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res 2004; 94: 1579–1588.
Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA 2002; 99: 10730–10735.
Kim YM, An JJ, Jin YJ, Rhee Y, Cha BS, Lee HC et al. Assessment of the anti-obesity effects of the TNP-470 analog, CKD-732. J Mol Endocrinol 2007; 38: 455–465.
Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR . Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 2005; 146: 4545–4554.
Lijnen HR, Christiaens V, Scroyen I, Voros G, Tjwa M, Carmeliet P et al. Impaired adipose tissue development in mice with inactivation of placental growth factor function. Diabetes 2006; 55: 2698–2704.
Silha JV, Krsek M, Sucharda P, Murphy LJ . Angiogenic factors are elevated in overweight and obese individuals. Int J Obes 2005; 29: 1308–1314.
Rega G, Kaun C, Demyanets S, Pfaffenberger S, Rychli K, Hohensinner PJ et al. Vascular endothelial growth factor is induced by the inflammatory cytokines interleukin-6 and oncostatin M in human adipose tissue in vitro and in murine adipose tissue in vivo. Arterioscler Thromb Vasc Biol 2007; 27: 1587–1595.
Fain JN, Madan AK . Insulin enhances vascular endothelial growth factor, interleukin-8, and plasminogen activator inhibitor 1 but not interleukin-6 release by human adipocytes. Metabolism 2005; 54: 220–226.
Okada S, Lin L, York DA, Bray GA . Chronic effects of intracerebral ventricular enterostatin in Osborne–Mendel rats fed a high-fat diet. Physiol Behav 1993; 54: 325–329.
Lin L, Chen J, York DA . Chronic ICV enterostatin preferentially reduced fat intake and lowered body weight. Peptides 1997; 18: 657–661.
Acknowledgements
This study was supported by funding from NIDDK 45278 and from the Utah Science, Technology and Research (USTAR) initiative to DAY.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, M., Lyons, J., Oh, H. et al. Enterostatin inhibition of angiogenesis: possible role of pAMPK and vascular endothelial growth factor A (VEGF-A). Int J Obes 32, 922–929 (2008). https://doi.org/10.1038/ijo.2008.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.16