Abstract
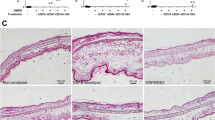
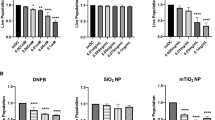
Contact hypersensitivity (CHS) is a delayed-type hypersensitivity that can be induced by haptens, such as 2,4-dinitrofluorobenzene (DNFB). Innate and adaptive immunities are both important for the development of CHS. To treat CHS-related diseases, such as allergic contact dermatitis, a disease prevalent in industrialized countries, ways of interfering with improper immune function during CHS responses need to be identified. Transforming growth factor-β-activated kinase-1 (TAK1), a member of mitogen-activated protein kinase kinase kinase family, is important for both innate and adaptive immunities. We thus hypothesized that the CHS response could be inhibited by interfering with TAK1 activity. Using a mouse model in which TAK1 deletion can be locally induced, we observed that TAK deficiency led to an impaired CHS response and was associated with defective T-cell expansion, activation and interferon (IFN)-γ production. In addition, we investigated the effect of deleting TAK1 specifically in dendritic cells (DC) on the CHS response. We found that when TAK1 is deficient in DC, the CHS response was abolished and hapten-elicited T-cell responses were defective. Collectively, this study demonstrates an essential role of TAK1 in the induction of CHS and suggests that targeting TAK1 could be a viable approach to treat CHS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Coenraads PJ, Goncalo M . Skin diseases with high public health impact. Contact dermatitis. Eur J Dermatol 2007; 17: 564–565.
Asano Y, Makino T, Norisugi O, Shimizu T . Occupational cobalt induced systemic contact dermatitis. Eur J Dermatol 2009; 19: 166–167.
Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D et al. Allergic contact dermatitis. Eur J Dermatol 2004; 14: 284–295.
Martin SF, Jakob T . From innate to adaptive immune responses in contact hypersensitivity. Curr Opin Allergy Clin Immunol 2008; 8: 289–293.
Jakob T, Ring J, Udey MC . Multistep navigation of Langerhans/dendritic cells in and out of the skin. J Allergy Clin Immunol 2001; 108: 688–696.
Xu H, DiIulio NA, Fairchild RL . T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med 1996; 183: 1001–1012.
Trautmann A, Akdis M, Schmid-Grendelmeier P, Disch R, Brocker EB, Blaser K et al. Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J Allergy Clin Immunol 2001; 108: 839–846.
Akiba H, Kehren J, Ducluzeau MT, Krasteva M, Horand F, Kaiserlian D et al. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+ T cytotoxic 1 cells inducing keratinocyte apoptosis. J Immunol 2002; 168: 3079–3087.
Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 2005; 6: 1087–1095.
Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev 2005; 19: 2668–2681.
Skaug B, Jiang X, Chen ZJ . The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem 2009; 78: 769–796.
Wan YY, Chi H, Xie M, Schneider MD, Flavell RA . The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol 2006; 7: 851–858.
Davis RJ . Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–252.
Schuman J, Chen Y, Podd A, Yu M, Liu HH, Wen R et al. A critical role of TAK1 in B-cell receptor-mediated nuclear factor kappaB activation. Blood 2009; 113: 4566–4574.
Landstrom M . The TAK1–TRAF6 signalling pathway. Int J Biochem Cell Biol 2010; 42: 585–589.
Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K . Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med 2005; 202: 1471–1476.
Vooijs M, Jonkers J, Berns A . A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep 2001; 2: 292–297.
Nosbaum A, Vocanson M, Rozieres A, Hennino A, Nicolas JF . Allergic and irritant contact dermatitis. Eur J Dermatol 2009; 19: 325–332.
Vocanson M, Hennino A, Cluzel-Tailhardat M, Saint-Mezard P, Benetiere J, Chavagnac C et al. CD8+ T cells are effector cells of contact dermatitis to common skin allergens in mice. J Invest Dermatol 2006; 126: 815–820.
Martin S, Lappin MB, Kohler J, Delattre V, Leicht C, Preckel T et al. Peptide immunization indicates that CD8+ T cells are the dominant effector cells in trinitrophenyl-specific contact hypersensitivity. J Invest Dermatol 2000; 115: 260–266.
Traidl C, Sebastiani S, Albanesi C, Merk HF, Puddu P, Girolomoni G et al. Disparate cytotoxic activity of nickel-specific CD8+ and CD4+ T cell subsets against keratinocytes. J Immunol 2000; 165: 3058–3064.
Vocanson M, Hennino A, Chavagnac C, Saint-Mezard P, Dubois B, Kaiserlian D et al. Contribution of CD4+ and CD8+ T-cells in contact hypersensitivity and allergic contact dermatitis. Expert Rev Clin Immunol 2005; 1: 75–86.
Martin SF, Dudda JC, Bachtanian E, Lembo A, Liller S, Durr C et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med 2008; 205: 2151–2162.
Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D et al. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J Biol Chem 2003; 278: 48928–48934.
Hammaker DR, Boyle DL, Chabaud-Riou M, Firestein GS . Regulation of c-Jun N-terminal kinase by MEKK-2 and mitogen-activated protein kinase kinase kinases in rheumatoid arthritis. J Immunol 2004; 172: 1612–1618.
Hammaker DR, Boyle DL, Inoue T, Firestein GS . Regulation of the JNK pathway by TGF-beta activated kinase 1 in rheumatoid arthritis synoviocytes. Arthritis Res Ther 2007; 9: R57.
Kimber I, Dearman RJ . Investigation of lymph node cell proliferation as a possible immunological correlate of contact sensitizing potential. Food Chem Toxicol 1991; 29: 125–129.
Gerberick GF, Cruse LW, Miller CM, Sikorski EE, Ridder GM . Selective modulation of T cell memory markers CD62L and CD44 on murine draining lymph node cells following allergen and irritant treatment. Toxicol Appl Pharmacol 1997; 146: 1–10.
Vocanson M, Hennino A, Rozieres A, Poyet G, Nicolas JF . Effector and regulatory mechanisms in allergic contact dermatitis. Allergy 2009; 64: 1699–1714.
Jiang Z, Zamanian-Daryoush M, Nie H, Silva AM, Williams BR, Li X . Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3–TRAF6–TAK1–TAB2–PKR. J Biol Chem 2003; 278: 16713–16719.
Irie T, Muta T, Takeshige K . TAK1 mediates an activation signal from Toll-like receptor(s) to nuclear factor-kappaB in lipopolysaccharide-stimulated macrophages. FEBS Lett 2000; 467: 160–164.
Zanoni I, Granucci F . Regulation of antigen uptake, migration, and lifespan of dendritic cell by Toll-like receptors. J Mol Med 2010; 88: 873–880.
Thiefes A, Wolter S, Mushinski JF, Hoffmann E, Dittrich-Breiholz O, Graue N et al. Simultaneous blockade of NFkappaB, JNK, and p38 MAPK by a kinase-inactive mutant of the protein kinase TAK1 sensitizes cells to apoptosis and affects a distinct spectrum of tumor necrosis factor [corrected] target genes. J Biol Chem 2005; 280: 27728–27741.
Wan YY, Flavell RA . TGF-beta and regulatory T cell in immunity and autoimmunity. J Clin Immunol 2008; 28: 647–659.
Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H . Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol 2005; 6: 1219–1227.
Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D . Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood 2003; 102: 3295–3301.
Acknowledgements
This work was supported by the National Institutes of Health (R00AI072956 to YYW), the Beijing Natural Science Foundation (7082054), the National Science and Technology Major Specific Project of the People's Republic of China (2009ZX09301-010 to WH) and the China Scholarship Council (YGZ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors have no conflicting interests.
Rights and permissions
About this article
Cite this article
Zhao, Y., Wang, Y., Hao, W. et al. An essential role for TAK1 in the contact hypersensitivity response. Cell Mol Immunol 8, 315–324 (2011). https://doi.org/10.1038/cmi.2011.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2011.11
Keywords
This article is cited by
-
Genetic dissection of dendritic cell homeostasis and function: lessons from cell type-specific gene ablation
Cellular and Molecular Life Sciences (2014)