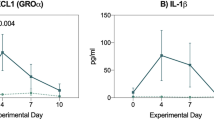
Abstract
Conditioning therapy in connection with haematopoietic SCT (HSCT) induces a disruption of the intestinal barrier function facilitating the permeation of bacteria and endotoxin through the bowel wall with subsequent increased risk of septicaemia and a worsening of GVHD in the allogeneic setting. Palifermin (recombinant human keratinocyte growth factor) reduces the severity of oral mucositis with HSCT. The present trial investigates its effect on intestinal barrier function. Seventeen lymphoma patients undergoing autologous HSCT received palifermin. Intestinal permeability was assessed before the conditioning therapy and on days +4 and +14. Clinical oral and gastrointestinal toxicity was prospectively assessed in parallel. A comparison was made with matched historical study patients (n=21). Patients treated with palifermin had a significantly better preserved intestinal barrier function (P=0.01 on day +4) and were in less need of total parenteral nutrition (P=0.005) as compared with controls. No significant reduction of clinical gastrointestinal or oral toxicity was observed. The intestinal barrier function, normally disrupted by the conditioning therapy, is preserved by palifermin. Whether intestinal barrier preservation protects from invasive infections, and in the allogeneic setting diminishes GVHD severity, remains to be investigated in randomized controlled trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Johansson JE, Ekman T . Gastro-intestinal toxicity related to bone marrow transplantation: disruption of the intestinal barrier precedes clinical findings. Bone Marrow Transplant 1997; 19: 921–925.
Fegan C, Poynton CH, Whittaker JA . The gut mucosal barrier in bone marrow transplantation. Bone Marrow Transplant 1990; 5: 373–377.
Clarkson JE, Worthington HV, Eden OB . Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2007. CD001973.
Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomb KL et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res 1998; 58: 933–939.
Farrell CL, Rex KL, Kaufman SA, DiPalma CR, Chen JN, Scully S et al. Effects of keratinocyte growth factor in the squamous epithelium of the upper aerodigestive tract of normal and irradiated mice. Int J Radiat Biol 1999; 75: 609–620.
Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med 2004; 351: 2590–2598.
Johansson JE, Abrahamsson H, Ekman T . Gastric emptying after autologous haemopoietic stem-cell transplantation: a prospective trial. Bone Marrow Transplant 2003; 32: 815–819.
Bjarnason I, O’Morain C, Levi AJ, Peters TJ . Absorption of 51chromium-labeled ethylenediaminetetraacetate in inflammatory bowel disease. Gastroenterology 1983; 85: 318–322.
Miller AB, Hoogstraten B, Staquet M, Winkler A . Reporting results of cancer treatment. Cancer 1981; 47: 207–214.
Aabakken L . Cr-ethylenediaminetetraacetic acid absorption test. Methodologic aspects. Scand J Gastroenterol 1989; 24: 351–358.
Berg RD, Garlington AW . Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun 1979; 23: 403–411.
Holler E, Kolb HJ, Mittermuller J, Kaul M, Ledderose G, Duell T et al. Modulation of acute graft-versus-host-disease after allogeneic bone marrow transplantation by tumor necrosis factor alpha (TNF alpha) release in the course of pretransplant conditioning: role of conditioning regimens and prophylactic application of a monoclonal antibody neutralizing human TNF alpha (MAK 195F). Blood 1995; 86: 890–899.
Holler E, Kolb HJ, Moller A, Kempeni J, Liesenfeld S, Pechumer H et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation [see comments]. Blood 1990; 75: 1011–1016.
Antin JH, Weinstein HJ, Guinan EC, McCarthy P, Bierer BE, Gilliland DG et al. Recombinant human interleukin-1 receptor antagonist in the treatment of steroid-resistant graft-versus-host disease. Blood 1994; 84: 1342–1348.
Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB . Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2- incompatible transplanted SCID mice. Blood 1994; 83: 2360–2367.
Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL . Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 1997; 90: 3204–3213.
Hill GR, Teshima T, Gerbitz A, Pan L, Cooke KR, Brinson YS et al. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest 1999; 104: 459–467.
Nestel FP, Price KS, Seemayer TA, Lapp WS . Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med 1992; 175: 405–413.
Storb R, Prentice RL, Buckner CD, Clift RA, Appelbaum F, Deeg J et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med 1983; 308: 302–307.
Panoskaltsis-Mortari A, Lacey DL, Vallera DA, Blazar BR . Keratinocyte growth factor administered before conditioning ameliorates graft-versus-host disease after allogeneic bone marrow transplantation in mice. Blood 1998; 92: 3960–3967.
Hill GR, Cooke KR, Teshima T, Crawford JM, Keith Jr JC, Brinson YS et al. Interleukin-11 promotes T cell polarization and prevents acute graft- versus-host disease after allogeneic bone marrow transplantation. J Clin Invest 1998; 102: 115–123.
Krijanovski OI, Hill GR, Cooke KR, Teshima T, Crawford JM, Brinson YS et al. Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood 1999; 94: 825–831.
Johansson JE, Ekman T . Gut toxicity during hemopoietic stem cell transplantation may predict acute graft-versus-host disease severity in patients. Dig Dis Sci 2007; 52: 2340–2345.
Nasilowska-Adamska B, Rzepecki P, Manko J, Czyz A, Markiewicz M, Federowicz I et al. The influence of palifermin (Kepivance) on oral mucositis and acute graft versus host disease in patients with hematological diseases undergoing hematopoietic stem cell transplant. Bone Marrow Transplant 2007; 40: 983–988.
Blazar BR, Weisdorf DJ, Defor T, Goldman A, Braun T, Silver S et al. Phase 1/2 randomized, placebo-control trial of palifermin to prevent graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). Blood 2006; 108: 3216–3222.
Acknowledgements
We thank all the patients for their participation in the trial. The study was supported by an unrestricted grant from Amgen. The company did not have any influence on the planning or conduct of the study and was not involved in the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johansson, JE., Hasséus, B., Johansson, P. et al. Gut protection by palifermin during autologous haematopoietic SCT. Bone Marrow Transplant 43, 807–811 (2009). https://doi.org/10.1038/bmt.2008.388
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.388
Keywords
This article is cited by
-
Systematic review of growth factors and cytokines for the management of oral mucositis in cancer patients and clinical practice guidelines
Supportive Care in Cancer (2020)
-
Systematic review of agents for the management of cancer treatment-related gastrointestinal mucositis and clinical practice guidelines
Supportive Care in Cancer (2019)
-
Impact of palifermin on intestinal mucositis of HSCT recipients after BEAM
Bone Marrow Transplantation (2014)
-
Systematic review of agents for the management of gastrointestinal mucositis in cancer patients
Supportive Care in Cancer (2013)
-
Systematic review of cytokines and growth factors for the management of oral mucositis in cancer patients
Supportive Care in Cancer (2013)