Abstract
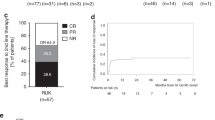
Budesonide (BUD) is a steroid with a low bioavailability, which has been used for the treatment of oral manifestations of chronic GVHD (cGVHD). We retrospectively evaluated the efficacy of BUD in the treatment of gastrointestinal cGVHD. Thirteen patients (median age 47 years) receiving BUD for the treatment of cGVHD after allogeneic hematopoietic SCT for hematological malignancies were evaluated for response. Five patients had isolated gastrointestinal cGVHD and 8 patients had mild multiorgan involvement including gastrointestinal manifestations. Six patients received CYA at the time of onset of cGVHD, which was continued during treatment with BUD. Treatment consisted of BUD, with an initial daily dose of 3 × 3 mg orally. Complete resolution of cGVHD was achieved in seven patients, and one patient achieved partial remission of cGVHD. One patient achieved complete resolution of gastrointestinal cGVHD, while systemic manifestations of cGVHD remained stable. Four patients progressed on BUD. Owing to the predominantly local effect, relapse of symptoms of cGVHD after withdrawal of immunosuppression (n=3) as well as progression of GVHD at other sites (n=3) has been observed. BUD represents a treatment option in mild-to-moderate cGVHD, which is well tolerated and associated with a high response rate in gastrointestinal cGVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood 2002; 100: 406–414.
Jacobsohn DA, Margolis J, Doherty J, Anders V, Vogelsang GB . Weight loss and malnutrition in patients with chronic graft-versus-host disease. Bone Marrow Transplant 2002; 29: 231–236.
Patey-Mariaud dS, Reijasse D, Verkarre V, Canioni D, Colomb V, Haddad E et al. Chronic intestinal graft-versus-host disease: clinical, histological and immunohistochemical analysis of 17 children. Bone Marrow Transplant 2002; 29: 223–230.
Sale GE, Shulman HM, McDonald GB, Thomas ED . Gastrointestinal graft-versus-host disease in man. A clinicopathologic study of the rectal biopsy. Am J Surg Pathol 1979; 3: 291–299.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Snover DC, Weisdorf SA, Vercellotti GM, Rank B, Hutton S, McGlave P . A histopathologic study of gastric and small intestinal graft-versus-host disease following allogeneic bone marrow transplantation. Hum Pathol 1985; 16: 387–392.
Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease—II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006; 12: 31–47.
Vogelsang GB, Wolff D, Altomonte V, Farmer E, Morison WL, Corio R et al. Treatment of chronic graft-versus-host disease with ultraviolet irradiation and psoralen (PUVA). Bone Marrow Transplant 1996; 17: 1061–1067.
Wolff D, Anders V, Corio R, Horn T, Morison WL, Farmer E et al. Oral PUVA and topical steroids for treatment of oral manifestations of chronic graft-vs-host disease. Photodermatol Photoimmunol Photomed 2004; 20: 184–190.
Bertz H, Afting M, Kreisel W, Duffner U, Greinwald R, Finke J . Feasibility and response to budesonide as topical corticosteroid therapy for acute intestinal GVHD. Bone Marrow Transplant 1999; 24: 1185–1189.
Elad S, Or R, Garfunkel AA, Shapira MY . Budesonide: a novel treatment for oral chronic graft versus host disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 95: 308–311.
McDonald GB, Bouvier M, Hockenbery DM, Stern JM, Gooley T, Farrand A et al. Oral beclomethasone dipropionate for treatment of intestinal graft-versus-host disease: a randomized, controlled trial. Gastroenterology 1998; 115: 28–35.
Hockenbery DM, Cruickshank S, Rodell TC, Gooley T, Schuening F, Rowley S et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood 2007; 109: 4557–4563.
Casper J, Knauf W, Kiefer T, Wolff D, Steiner B, Hammer U et al. Treosulfan and fludarabine: a new toxicity-reduced conditioning regimen for allogeneic hematopoietic stem cell transplantation. Blood 2004; 103: 725–731.
Schmid C, Schleuning M, Hentrich M, Markl GE, Gerbitz A, Tischer J et al. High antileukemic efficacy of an intermediate intensity conditioning regimen for allogeneic stem cell transplantation in patients with high-risk acute myeloid leukemia in first complete remission. Bone Marrow Transplant 2008; 41: 721–727.
Wolff D, Roessler V, Steiner B, Wilhelm S, Weirich V, Brenmoehl J et al. Treatment of steroid-resistant acute graft-versus-host disease with daclizumab and etanercept. Bone Marrow Transplant 2005; 35: 1003–1010.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood 2002; 100: 48–51.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andree, H., Hilgendorf, I., Leithaeuser, M. et al. Enteral budesonide in treatment for mild and moderate gastrointestinal chronic GVHD. Bone Marrow Transplant 42, 541–546 (2008). https://doi.org/10.1038/bmt.2008.209
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.209
Keywords
This article is cited by
-
A prospective evaluation on the interaction of fluconazole and voriconazole on serum concentrations of budesonide in patients treated for gastrointestinal GVHD
Bone Marrow Transplantation (2020)
-
Preparation and characterization of solid lipid nanoparticle-based nasal spray of budesonide
Drug Delivery and Translational Research (2013)
-
The EBMT Paediatric Diseases Working Party: current concepts and future aims
memo - Magazine of European Medical Oncology (2009)