Abstract
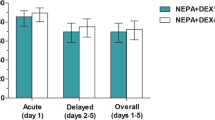
Thirty-three patients with lung cancer receiving 80 mg m(-2) cisplatin were treated with high-dose dexamethasone (32 mg m(-2) on days 1-3, 16 mg m(-2) on day 4 and 8 mg m(-2) on day 5) combined with granisetron on day 1 and metoclopramide on days 2-5. Twenty-eight (85%) patients had no nausea or vomiting on day 1, and 16 (48%) achieved total control on days 1-5 with acceptable toxicity. High-dose dexamethasone for cisplatin-induced delayed emesis should be further evaluated in a phase III trial.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sekine, I., Nishiwaki, Y., Kakinuma, R. et al. Phase II study of high-dose dexamethasone-based association in acute and delayed high-dose cisplatin-induced emesis – JCOG study 9413. Br J Cancer 76, 90–92 (1997). https://doi.org/10.1038/bjc.1997.341
Issue Date:
DOI: https://doi.org/10.1038/bjc.1997.341
This article is cited by
-
Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: Update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis
International Journal of Clinical Oncology (2021)
-
Japanese Society of Clinical Oncology clinical practice guidelines 2010 for antiemesis in oncology: executive summary
International Journal of Clinical Oncology (2016)
-
Low-dose dexamethasone effectively prevents postoperative nausea and vomiting after ambulatory laparoscopic surgery
Canadian Journal of Anesthesia/Journal canadien d'anesthésie (2001)