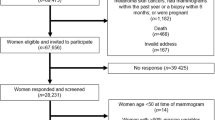
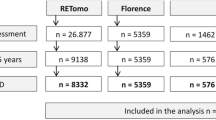
Abstract
The most convincing evidence that a factor such as dietary fat is causally related to breast cancer would be obtained from a randomised controlled trial in which exposure to dietary fat intake was systematically varied. A limitation of randomised controlled trials of breast cancer prevention, however, is the large sample size required to detect plausible reductions in risk resulting from the intervention. We describe here experience over a period of 9 years with the use of one risk factor for breast cancer as a criterion for entry to a clinical trial of breast cancer prevention. The risk factor used was the presence of extensive densities in the breast tissue on mammography, which has been found by several investigators to be strongly associated with risk of breast cancer. Using this criterion for selection, 1800 subjects of mean age 46 years were enrolled between 1982 and 1986, and again between 1988 and the present. Throughout this period, the point estimate of annual invasive cancer incidence was approximately 6 per 1000 per year. The observed cancer incidence has been consistently 4-5 times the incidence expected from age-specific breast cancer incidence data for women living in Ontario. These data show that the selection of subjects for a clinical trial of breast cancer prevention using the criterion of extensive breast parenchymal densities does identify a group at substantially increased risk of breast cancer. Use of this criterion for the selection of subjects can substantially reduce the sample size required for a clinical trial of a preventive strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boyd, N., Fishell, E., Jong, R. et al. Mammographic densities as a criterion for entry to a clinical trial of breast cancer prevention. Br J Cancer 72, 476–479 (1995). https://doi.org/10.1038/bjc.1995.358
Issue Date:
DOI: https://doi.org/10.1038/bjc.1995.358
This article is cited by
-
Association between menopausal hormone therapy, mammographic density and breast cancer risk: results from the E3N cohort study
Breast Cancer Research (2021)
-
Suitable trial designs and cohorts for preventive breast cancer agents
Nature Reviews Clinical Oncology (2013)
-
Effect of a low-fat, high-carbohydrate dietary intervention on change in mammographic density over menopause
Breast Cancer Research and Treatment (2009)