Abstract
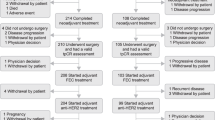
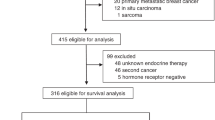
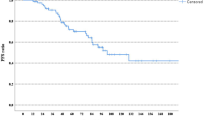
A randomised clinical trial has been conducted to compare adjuvant tamoxifen, 20 mg daily, with tamoxifen and prednisolone, 7.5 mg daily, in post-menopausal women with operable breast cancer. There were 254 evaluable patients, of whom 128 were given tamoxifen alone and 126 received tamoxifen and prednisolone. After a median follow-up of 48 months there was no significant difference in relapse-free or overall survival of the two groups. Furthermore, with survival slightly favouring tamoxifen, confidence intervals on the hazard ratio established that a difference in favour of tamoxifen plus prednisolone of even 5% at 5 years was very unlikely (P < 0.02). Thus, despite the relatively small number of patients in this trial, the data clearly establish that prednisolone is not of value as an additional adjuvant agent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fentiman, I., Howell, A., Hamed, H. et al. A controlled trial of adjuvant tamoxifen, with or without prednisolone, in post-menopausal women with operable breast cancer. Br J Cancer 70, 729–731 (1994). https://doi.org/10.1038/bjc.1994.384
Issue Date:
DOI: https://doi.org/10.1038/bjc.1994.384
This article is cited by
-
The Immunomodulatory Effects of Dexamethasone on Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer
Oncology and Therapy (2023)
-
Effect of glucocorticoid use on survival in patients with stage I–III breast cancer
Breast Cancer Research and Treatment (2018)
-
Systematic review of the clinical effect of glucocorticoids on nonhematologic malignancy
BMC Cancer (2008)
-
The influence on survival of delay in the presentation and treatment of symptomatic breast cancer
British Journal of Cancer (1999)