Abstract
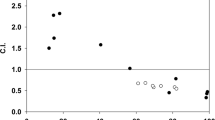
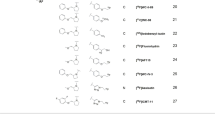
Eight anthracycline analogs that have been shown to modulate multidrug resistance (Friche et al., Biochem. Pharmacol., 39, 1721-1726; 1990) were tested for their inhibitory effect on the photolabelling of P-glycoprotein. We photoaffinity labelled P-glycoprotein in daunorubicin (DNR) resistant Ehrlich ascites tumour cells (EHR2/DNR +) with a [125I]iodinated Bolton-Hunter derivative of daunorubicin ([125I]iodomycin) and with [3H]azidopine. The photolabelling of P-glycoprotein by [125I]iodomycin was inhibited more than 50% by 10 microM (1000-fold molar excess) of DNR (52%), N,N-dibenzyl-DNR (52%), and N-benzyladriamycin-14-valerate (AD-198) (85%). Vincristine at 10 microM inhibited [125I]iodomycin labelling of P-glycoprotein by 95%. Thus vincristine was more potent than any of the eight anthracyclines tested, despite its relatively low lipophilicity. Increasing the concentration of DNR, AD-198 and N,N-dibenzyl-DNR to 40 microM resulted in 90, 99.5 and 99.5% inhibition of P-glycoprotein labelling by [125I]iodomycin, respectively. In comparison with the other anthracycline analogs, N,N-dibenzyl-DNR and Ad-198 were also found to exert the greatest inhibition of [3H]azidopine labelling of P-glycoprotein (about 90% at 100-fold molar excess). The solvents Cremophor EL and Tween 80 (30 micrograms ml-1; 0.003% v/v), which are modulators of multidrug resistance in EHR2/DNR + cells, also inhibited [125I]iodomycin labelling > 90%. We showed earlier that there is a correlation between the lipid solubility within the anthracycline group of MDR-associated drugs and their ability to enhance DNR accumulation in EHR2/DNR + cells but a corresponding correlation to lipophilicity when it comes to the inhibitory effect on the specific photolabelling of Pgp ligand binding sites could not be demonstrated. Neither could a correlation between the modulating effect of the analogs on DNR accumulation and inhibition on the labelling of Pgp be demonstrated. With increasing lipophilicity of the analogs it seems that the chemical structure plays a lesser role, and the degree of lipophilicity becomes a more important feature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Friche, E., Demant, E., Sehested, M. et al. Effect of anthracycline analogs on photolabelling of p-glycoprotein by [125I]iodomycin and [3H]azidopine: relation to lipophilicity and inhibition of daunorubicin transport in multidrug resistant cells. Br J Cancer 67, 226–231 (1993). https://doi.org/10.1038/bjc.1993.44
Issue Date:
DOI: https://doi.org/10.1038/bjc.1993.44
This article is cited by
-
A new approach for simultaneous calculation of pIC50 and logP through QSAR/QSPR modeling on anthracycline derivatives: a comparable study
Journal of the Iranian Chemical Society (2021)
-
The “Multi” of Drug Resistance Explained by Oscillating Drug Transporters, Drug–Membrane Physical Interactions and Spatial Dimensionality
Cell Biochemistry and Biophysics (2011)
-
Cytotoxicity of fractionated paclitaxel (Taxol®) administration in vitro
Strahlentherapie und Onkologie (1998)