Abstract
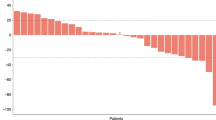
A phase I study was undertaken in order to establish the maximum tolerated dose of intra-hepatic arterial 5-fluorouracil (5-FU) when given in combination with systemic folinic acid. Patients with colorectal liver metastases (n = 10) received escalating doses of 5-FU as a 24 h infusion with a fixed dose (400 mg m-2) of intravenous folinic acid once per week. Dose limiting toxicity (WHO grade greater than 2) was encountered at 2 g m-2 5-FU. Principal adverse effects were diarrhoea, vomiting and oral ulceration. The recommended dose for phase II studies is 1.5 g m-2 week-1 24 h 5-FU regional infusion with 400 mg m-2 week-1 intravenous folinic acid.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anderson, J., Kerr, D., Cooke, T. et al. A phase I study of regional 5-fluorouracil and systemic folinic acid for patients with colorectal liver metastases. Br J Cancer 65, 913–915 (1992). https://doi.org/10.1038/bjc.1992.191
Issue Date:
DOI: https://doi.org/10.1038/bjc.1992.191