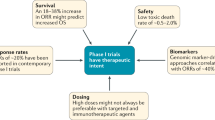
Abstract
Potential anti-cancer agents have classically undergone clinical assessment in Phase I, II and III trials. This paper examines the role of these trials and pre-clinical studies in the light of improving cancer chemotherapy. Many patients must now be treated with standard therapy before investigational drugs can be ethically used. The introduction of combined modality trials will require a very prolonged follow-up to demonstrate improved survival and recognize late onset of chronic toxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Rights and permissions
About this article
Cite this article
Williams, C., Carter, S. Management of trials in the development of cancer chemotherapy. Br J Cancer 37, 434–447 (1978). https://doi.org/10.1038/bjc.1978.64
Issue Date:
DOI: https://doi.org/10.1038/bjc.1978.64
This article is cited by
-
Phase I evaluation of divided-dose vinblastine sulfate
Cancer Chemotherapy and Pharmacology (1980)