Abstract
Objective:
To determine the capillary partial pressure of carbon dioxide (PCO2) and room air transcutaneous hemoglobin saturation (RA SAT) at 36 weeks’ postmenstrual age (PMA) in infants born with weight between 501 and 1250 g.
Study Design:
Multicenter, prospective investigation with primary data collection within 72 h of 36 weeks PMA or discharge, whichever first. PCO2 and RA SAT determinations were done at rest on infants not requiring mechanical ventilation or nasal continuous positive airway pressure (NCPAP).
Result:
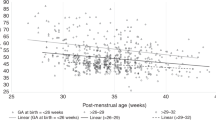
A total of 220 infants were enrolled (mean gestational age 27.7 weeks, mean birthweight 951 g). In infants with traditionally defined chronic lung disease (CLD) compared to those without CLD, the mean PCO2 was significantly higher (54 versus 45 mm Hg) and the median RA SAT significantly lower (<80 versus 97%). In infants with the new classification of bronchopulmonary dysplasia (BPD), there was a significant linear trend toward increasing PCO2 with increasing severity of BPD (45, 47, 54 and 62 mm Hg in No, Mild, Moderate and Severe BPD). There was a significant linear trend toward decreasing RA SAT with increasing severity of BPD (97, 95 <80, <80% in No, Mild, Moderate and Severe BPD).
Conclusion:
Defining CLD as BPD based upon a RA SAT test is a more discriminate, objective method to categorize lung injury. PCO2 is an objective measure of lung function that inversely correlates with RA SAT. These determinations done together at 36 weeks PMA may provide more precise and accurate estimates of lung injury that might allow for better understanding of pulmonary therapies and clearer comparison of BPD rates and severities among NICUs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vermont Oxford Network. Annual Report 2005. Vermont Oxford Network: Burlington, VT; Section 2, Table 2.4.
Hoekstra RE, Ferrara TB, Couser RJ, Payne NR, Connett JE . Survival and long-term neurodevelopmental outcome of extremely premature infants born at 23 to 26 weeks gestational age at a tertiary center. Pediatrics 2004; 113 (1): e1–e6.
Payne NR, LaCorte M, Karna P, Chen S, Finkelstein M, Goldsmith JP et al. Reduction of chronic lung disease after participation in the Breathsavers group of the Vermont Oxford Network Neonatal Intensive Care Quality Improvement Collaborative. Pediatrics 2006; 118 (Suppl 2): S73–S77.
Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 1987; 79 (1): 26–30.
Van Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? Pediatrics 2000; 105 (6): 1194–1201.
Ellsbury DL, Acarregui MJ, McGuiness GA, Klein JM . Variability in the use of supplemental oxygen for bronchopulmonary dysplasia. J Pediatr 2002; 140 (2): 247–249.
Jobe AH, Bancalari E . Bronchopulmonary dysplasia. NICHD/NHLBI/ORD workshop summary. Am J Respir Crit Care Med 2001; 163: 1723–1729.
Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004; 114 (5): 1305–1311.
Ryan RM . A new look at bronchopulmonary dysplasia classification. J Perinatol 2006; 26 (4): 207–209.
Kaempf JW, Campbell B, Sklar RS, Arduza C, Gallegos R, Zabari M et al. Implementing potentially better practices to improve neonatal outcomes after reducing postnatal dexamethasone use in infants born between 501 and 1250 grams. Pediatrics 2003; 111 (4): e534–e541.
Vermont Oxford Network Manual of Operations. Release 8.0. 2004, page 72. Vermont Oxford Network: Burlington, VT.
Madan A, Brozanski BS, Cole CH, Oden NL, Cohen G, Phelps DL . A pulmonary score for assessing the severity of neonatal chronic lung disease. Pediatrics 2005; 115 (4): e450–e457.
Walsh MC, Laptook A, Kazzi N, Engle WA, Yao Q, Rasmussen M et al. A cluster-randomized trial of benchmarking and multimodal quality improvement to improve rates of survival free of bronchopulmonary dysplasia for infants with birth weights of less than 1250 grams. Pediatrics 2007; 119 (5): 876–890.
Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005; 116 (6): 1353–1360.
Sahni R, Ammari A, Suri MS, Milisavljevic V, Ohira-Kist K, Wung JT et al. Is the new definition of bronchopulmonary dysplasia more useful? J Perinatol 2005; 25 (1): 41–46.
Johnson KJ, Cress GA, Connolly NW, Burmeister LF, Widness JA . Neonatal laboratory blood sampling: comparison of results from arterial catheters with those from an automated capillary device. Neonatal Netw 2000; 19 (1): 27–34.
Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM . Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med 2003; 349 (10): 959–967.
Chow LC, Wright KW, Sola A . Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics 2003; 111 (2): 339–345.
Kovesi T, Abdurahman A, Blayney M . Elevated carbon dioxide tension as a predictor of subsequent adverse events in infants with bronchopulmonary dysplasia. Lung 2006; 184: 7–13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaempf, J., Campbell, B., Brown, A. et al. PCO2 and room air saturation values in premature infants at risk for bronchopulmonary dysplasia. J Perinatol 28, 48–54 (2008). https://doi.org/10.1038/sj.jp.7211859
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211859
Keywords
This article is cited by
-
A modified physiologic test for bronchopulmonary dysplasia: a clinical tool for weaning from CPAP and/or oxygen-therapy the premature babies?
Italian Journal of Pediatrics (2019)
-
Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants
Journal of Perinatology (2011)