Abstract
Objective:
Inhaled nitric oxide (iNO) use in infants >1500 g, but <34 weeks gestation with severe respiratory failure will reduce the incidence of death and/or bronchopulmonary dysplasia (BPD).
Study Design:
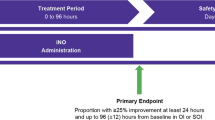
Infants born at <34 weeks gestation with a birth weight >1500 g with respiratory failure were randomly assigned to receive placebo or iNO.
Results:
Twenty-nine infants were randomized. There were no differences in baseline characteristics, but the status at randomization showed a statistically significant difference in the use of high-frequency ventilation (P=0.03). After adjustment for oxygenation index entry strata, there was no difference in death and/or BPD (adjusted relative risk (RR) 0.80, 95% confidence interval (CI) 0.43 to 1.48; P=0.50), death (adjusted RR 1.26, 95% CI 0.47 to 3.41; P=0.65) or BPD (adjusted RR 0.40, 95% CI 0.47 to 3.41; P=0.21).
Conclusions:
Although sample size limits our ability to make definitive conclusions, this small pilot trial of iNO use in premature infants >1500 g and <34 weeks with severe respiratory failure suggests that iNO does not affect the rate of BPD and/or death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics 2001; 107: E1.
St. John EB, Carlo WA . Respiratory distress syndrome in VLBW infants: Changes in management and outcomes observed by the NICHD Neonatal Research Network. Semin Perinatol 2003; 27: 288–292.
The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 1997; 336: 597–604.
Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. N Engl J Med 2000; 342: 469–474.
Frostell C, Fratacci M-D, Wain JC, Jones R, Zapol WM . Inhaled nitric oxide, a selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991; 83: 2038–2047.
Martin RJ, Walsh MC . Inhaled nitric oxide for preterm infants – who benefits? N Engl J Med 2005; 353: 82–85.
Bland RD, Ling CY, Albertine KH, Carlton DP, MacRitchie AJ, Day RW et al. Pulmonary vascular dysfunction in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2003; 288: L1178–L1185.
Garg UC, Hassid A . Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 1989; 83: 1774–1777.
Thomae KR, Nakayama DK, Billiar TR, Simmons RL, Pitt BR, Daies P . The effect of nitric oxide on fetal pulmonary artery smooth muscle growth. J Surg Res 1995; 59: 457–463.
Roberts Jr JD, Roberts CT, Jones RC, Zapol WM, Bloch KD . Continuous nitric oxide inhalation reduces pulmonary arterial structural changes, right ventricular hypertrophy, and growth retardation in the hypoxic newborn rat. Circ Res 1995; 76: 215–222.
McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2005; 288: L450–L459.
Hamad AM, Johnson SR, Knox AJ . Antiproliferative effects of NO and ANP in cultured human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 1999; 277: L910–L918.
Bland RD, Albertine KH, Carlton DP, MacRitchie AJ . Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med 2005; 172: 899–906.
Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, Abman SH . Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res 1997; 41: 457–463.
Blatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM . Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 164: 1971–1980.
Lin Y-J, Markham NE, Balasubramaniam V, Tang J-R, Maxey A, Kinsella JP et al. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 2005; 58: 22–29.
Kinsella JP, Parker TA, Galan H . Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res 1997; 41: 457–463.
Subhedar NV, Ryan SW, Shaw NJ . Open randomized controlled trial of inhaled nitric oxide and early dexamethasone in high risk premature infants. Arch Dis Child 1997; 77: F185–F190.
Kinsella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S et al. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomized controlled trial. Lancet 1999; 354: 1061–1065.
The Franco-Belgium Collaborative Trial Group. Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomized controlled trial. Lancet 1999; 354: 1066–1071.
Hascoet JM, Fresson J, Claris O, Hamon I, Lombet J, Liska A et al. The safety and efficacy of nitric oxide therapy in premature infants. J Pediatr 2005; 146: 318–323.
Field D, Elbourne D, Truesdale A, Grieve R, Hardy P, Fenton AC et al. Neonatal ventilation with inhaled nitric oxide versus ventilatory support without inhaled nitric oxide for preterm infants with severe respiratory failure: the INNOVO multicentre randomized controlled trial. Pediatrics 2005; 115: 926–936.
Schreiber MD, Gin-Mestran K, Marks JD, Huo D, Lee G, Srisuparp P . Inhaled nitric oxide in premature infants with respiratory distress syndrome. N Engl J Med 2003; 349: 2099–2107.
Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med 2005; 353: 13–22.
Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart CL et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med 2006; 355: 354–364.
Walsh MC, Yao Q, Gettner P, Collins M, Hensman A, Everette R et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004; 114: 1305–1311.
Konduri GG, Solimano A, Sokol GM, Singer J, Ehrenkranz RA, Singhal N et al. Neonatal inhaled nitric oxide study group. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics 2004; 113: 559–564.
Zou G . A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–706.
Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD . Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. N Engl J Med 2005; 353: 23–32.
Hintz SR, Van Meurs KP . Neurodevelopmental outcomes of the NICHD randomized controlled trial of iNO for premature infants with severe respiratory failure. E-PAS 2006; 59: 2755.
Acknowledgements
Funded by National Institute of Child Health and Development (NICHD) and Department of Health and Human Services (DHHS) U10 HD34216, U10 HD27853, U10 HD27871, U10 HD40461, U10 HD40689, U10 HD27856, U10 HD27904, U10 HD40498, U10 HD40521, U01 HD36790, U10 HD21385, U10 HD27880, U10 HD27851, U10 HD 21373 and GCRCs: M01 RR 08084, M01 RR 06022, M01 RR 00750, M01 RR 00070, M01RR 00039, M01 RR00044. NO Therapeutics provided study gas, gas delivery systems, and site monitoring for all hospitals, as well as capitation funding for the hospitals that were not members of the NICHD Neonatal Research Network. INO Therapeutics was not involved in the study design, data analysis and interpretation, or paper preparation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Meurs, K., Hintz, S., Ehrenkranz, R. et al. Inhaled nitric oxide in infants >1500 g and <34 weeks gestation with severe respiratory failure. J Perinatol 27, 347–352 (2007). https://doi.org/10.1038/sj.jp.7211690
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211690
Keywords
This article is cited by
-
Inhaled nitric oxide in premature infants for preventing bronchopulmonary dysplasia: a meta-analysis
BMC Pediatrics (2023)
-
Use of inhaled nitric oxide in preterm vs term/near-term neonates with pulmonary hypertension: results of the PaTTerN registry study
Journal of Perinatology (2022)
-
Our paper 20 years later: Inhaled nitric oxide for the acute respiratory distress syndrome—discovery, current understanding, and focussed targets of future applications
Intensive Care Medicine (2014)
-
Systematic review of safety in paediatric drug trials published in 2007
European Journal of Clinical Pharmacology (2012)