Abstract
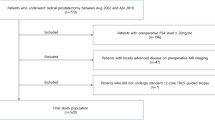
The objectives of this work were to evaluate the efficacy of controlled close step-sectioned and whole-mounted radical prostatectomy specimen processing in prediction of clinical outcome as compared to the traditional processing techniques. Two-hundred and forty nine radical prostatectomy (RP) specimens were whole-mounted and close step-sectioned at caliper-measured 2.2–2.3 mm intervals. A group of 682 radical prostatectomy specimens were partially sampled as control. The RPs were performed during 1993–1999 with a mean follow-up of 29.3 months, pretreatment PSA of 0.1–40, and biopsy Gleason sums of 5–8. Disease-free survival based on biochemical or clinical recurrence and secondary intervention were computed using a Kaplan-Meier analysis. There were no significant differences in age at diagnosis, age at surgery, PSA at diagnosis, or biopsy Gleason between the two groups (P<0.05). Compared with the non-close step-sectioned group, the close step-sectioned group showed higher detection rates of extra-prostatic extension (215 (34.1%) vs, 128 (55.4%), P<0.01), and seminal vesicle invasion (50 (7.6%) vs 35 (14.7%), P<0.01). The close step-sectioned group correlated with greater 3-y disease-free survival in organ-confined (P<0.01) and specimen-confined (P<0.01) cases, over the non-uniform group. The close step-sectioned group showed significantly higher disease-free survival for cases with seminal vesicle invasion (P=0.046). No significant difference in disease-free survival was found for the positive margin group (P=0.39) between the close step-sectioned and non-uniform groups. The close step-sectioned technique correlates with increased disease-free survival rates for organ and specimen confined cases, possibly due to higher detection rates of extra-prostatic extension and seminal vesicle invasion. Close step-sectioning provides better assurance of organ-confined disease, resulting in enhanced prediction of outcome by pathological (TNM) stage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Greenlee RT et al. Cancer statistics, 2001 CA Cancer J Clin 2001 51: 15–36
Blute ML et al. Pathological classification of prostate carcinoma: the impact of margin status Cancer 1998 82: 902
Epstein JI, Pizov G, Walsh PC . Correlation of pathologically findings with progression after a radical retropubic prostatectomy Cancer 1993 71: 3582
McNeal JE . Cancer volume and site of origin of adenocarcinoma in the prostate: relationship to local and distant spread Hum Path 1992 23: 258
Renshaw AA, Chang H, D'Amico AV . Estimation of tumor volume in radical prostatectomy specimens in routine clinical practice Am J Clin Pathol 1997 107: 704
Chen ME et al. Prostate cancer detection: relationship to prostate size Urology 1999 53: 764
Renshaw AA . Correlation of gross morphological features with histologic features in radical prostatectomy specimens Am J Clin Pathol 1998 110: 38
True LD . Surgical pathology examination of the prostate gland: practice survey of clinical pathologists Am J Clin Pathol 1994 102: 572
Hall GS, Kramer CE, Epstein JI . Evaluation of radical prostatectomy specimens: a comparative analysis of sampling methods Am J Surg Pathol 1992 16: 315
Cohen MB, Soloway MS, Murphy WM . Sampling of radical prostatectomy specimens: how much is adequate? Am J Clin Pathol 1994 101: 250
Epstein JI . Evaluation of radical prostatectomy capsular margins of resection: the significance of capsular margins designated as negative, closely approaching and positive Am J Surg Pathol 1990 12: 626
Donohue RE, Miller GJ . Adenocarcinoma of the prostate: biopsy to whole mount, Denver VA experience Urol Clin North Am 1991 18: 449
Hollenbeck BK et al. Whole mounted radical prostatectomy specimens do not increase detection of adverse pathological features J Urol 2000 164: 1583
Grossfeld GD et al. Does the completeness of prostate sampling predict outcome for patients undergoing radical prostatectomy? Data from the CaPSURE database Urology 2000 56: 430
Humphrey PA . Tissue sampling in the era of cost restraints Am J Clin Pathol 1994 101: 247
Byar DP, Mostofi FK . Carcinoma of the prostate: prognostic evaluation of certain pathological features in 208 radical prostatectomies examined by step-section technique Cancer 1972 30: 5
Epstein JI . The evaluation of radical prostatectomy specimens: therapeutic and prognostic implications Pathol Annu 1991 26: 159
Sakr WA et al. Evaluation of radical prostatectomy specimens: a practical approach for establishing tumor pathological stage Mod Pathol 1993 6: 68A
Schmid HP, McNeal JE . An abbreviated standard procedure for accurate tumor volume estimation in prostate cancer Am J Surg Pathol 1992 16: 184
Moul JW et al. Racial differences in tumor volume and prostate specific antigen among radical prostatectomy patients J Urol. 1999 162: 394
Mettlin CJ et al. The National Cancer Database report on increased use of brachytherapy for the treatment of patients with prostate carcinoma in the US Cancer 1999 86: 1877
Blute ML et al. Pathologic classification of prostate carcinoma: the impact of margin status Cancer 1997 82: 902
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as representing the views of the United States Army or Department of Defense.
This research was supported by the Department of Defense Center for Prostate Disease Research (CPDR), a program funded by the US Army Medical Research and Material Command (USAMRMC) through the Uniformed Services University (USU) and administered by the Henry M. Jackson Foundation for the Advancement of Military Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desai, A., Wu, H., Sun, L. et al. Complete embedding and close step-sectioning of radical prostatectomy specimens both increase detection of extra-prostatic extension, and correlate with increased disease-free survival by stage of prostate cancer patients. Prostate Cancer Prostatic Dis 5, 212–218 (2002). https://doi.org/10.1038/sj.pcan.4500600
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500600
Keywords
This article is cited by
-
Epidemiologie, Pathologie und Molekularbiologie des Prostatakarzinoms
Der Onkologe (2013)
-
Correlation of histomorphologic findings and partial neurovascular bundle preservation during laparoscopic and robotic radical prostatectomy
Journal of Robotic Surgery (2013)
-
Robotic-assisted laparoscopic prostatectomy
British Journal of Cancer (2009)
-
Offene, laparoskopische und roboterassistierte radikale Prostatektomie im Vergleich
Der Onkologe (2007)
-
Tissue print micropeel: A new technique for mapping tumor invasion in prostate cancer
Current Urology Reports (2006)