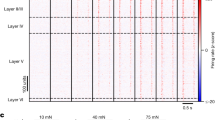
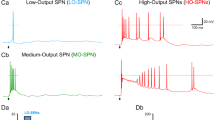
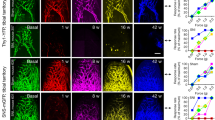
Abstract
AFTER peripheral nerve section, sensory neurons regenerate but do not regain their original topographical position in the skin1. Here we report that in the early stages of sciatic nerve regeneration, the cutaneous receptive fields (RFs) of dorsal horn neurons are larger than normal, reflecting the disorganized topography of the regenerated afferents. When nerve regeneration is complete, small contiguous RFs emerge, indicating a central compensation for the disrupted peripheral somatotopy. If the NMDA receptor antagonist MK801 is given during regeneration, RFs do not show this reorganization, but remain large and diffuse. We suggest that the coincident activity of afferents, newly innervating adjacent or over-lapping cutaneous territory, acts through postsynaptic NMDA receptors2 to strengthen the central effectiveness of these inputs at the expense of other non-adjacent and non-coincidently activated inputs. In this way, dorsal horn neurons may attain and retain restricted RFs in the face of a spatially dispersed afferent input.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fawcett, J. W. & Keynes, R. J. A. Rev. Neurosci. 13, 43–60 (1990).
Constantine-Paton, M., Cline, H. T. & Debeski, E. A. Rev. Neurosci. 13, 129–154 (1990).
Dykes, R. W. & Terzis, J. K. J. Neurophysiol. 42, 1461–1478 (1979).
Horch, K. J. Neurophysiol. 42, 1437–1449 (1979).
Devor, M. & Wall, P. D. Nature 276, 75–76 (1978).
Devor, M. & Wall, P. D. J. Neurosci. 1, 679–684 (1981).
Lisney, S. J. W. Brain Res. 259, 31–39 (1983).
McMahon, S. B., Lewin, G. R. & Wall, P. D. Curr. Opin. Neurobiol. 3, 602–610 (1993).
Tulle, T. R., Berthele, A., Zieglgansberger, W., Seeburg, P. H. & Wisden, W. J. Neurosci. 13, 5009–5028 (1993).
Wong, E. H. et al. Proc. natn. Acad. Sci. U.S.A. 83, 7104–7108 (1986).
Woolf, C. J. & Thompson, S. W. N. Pain 44, 293–299 (1991).
Hao, J. X., Aldskogius, H., Seiger, A. & Weisenfeld-Hallin, Z. Expl Neurology 113, 182–191 (1991).
Headley, P. M., Parsons, C. G. & West, D. C. J. Physiol. 385, 169–180 (1984).
Dougherty, P. M., Palecek, J., Paleckova, V., Sorkin, L. S. & Willis, W. D. J. Neurosci. 12, 3025–3041 (1992).
Davies, S. N. & Lodge, D. Brain Res. 424, 402–406 (1987).
Dickenson, A. H. & Sullivan, A. F. Neuropharmacology 26, 1235–1238 (1987).
Agrawal, S. G. & Evans, R. H. Br. J. Pharmac. 87, 345–355 (1986).
Huettner, J. E. Neuron 5, 255–266 (1990).
Li, Y., Erzurumlu, R. S., Chen, C., Jhaveri, S. & Tonegawa, S. Cell 76, 427–437 (1994).
Thompson, S. W. N., King, A. E. & Woolf, C. J. Eur. J. Neurosci. 2, 638–649 (1990).
Schaible, H.-G., Grubb, B. D., Neugebauer, V. & Oppmann, M. Eur. J. Neurosci. 3, 981–991 (1991).
Lewin, G. R. & McMahon, S. B. Eur. J. Neurosci. 5, 1083–1092 (1993).
Hebb, D. O. The Organisation of Behaviour (Wiley, New York, 1949).
Morris, R. G., Davis, S. & Butcher, S. P. Phil. Trans. R. Soc. Lon. B329, 187–204 (1990).
Watkins, J. C. The NMDA Receptor Concept: Origins and Development (eds Watkins, J. C. & Collingridge, G. L.) 1–19 (Oxford University Press, Oxford, 1989).
Randic, M., Jiang, M. C. & Cerne, R. J. Neurosci. 13, 5228–5241 (1993).
Kaas, J. H. A. Rev. Neurosci. 14, 137–167 (1991).
Kano, M., Lino, K. & Kano, M. NeuroReport 2, 77–80 (1991).
Shlagger, B. L., Fox, K. & O'Leary, D. D. M. Nature 364, 623–625 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lewin, G., Mckintosh, E. & McMahon, S. NMDA receptors and activity-dependent tuning of the receptive fields of spinal cord neurons. Nature 369, 482–485 (1994). https://doi.org/10.1038/369482a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/369482a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.