Abstract
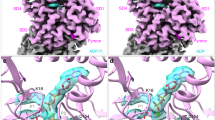
ELUCIDATION of the molecular contacts between actin and myosin is central to understanding the force-generating process in muscle and other cells. Actin, a highly conserved globular protein found in all eukaryotes, polymerizes into filaments (F-actin) for most of its biological functions. Myosins, which are more diverse in sequence, share a conserved globular head of about 900 amino acids in length (subfragment-1 or S1) at the N-terminal end of the molecule. S1 contains all the elements necessary for mechano-chemical force transduction in vitro1,2. Here we report an atomic model for the actomyosin complex produced by combining the atomic X-ray structure of F-actin3,4 and chicken myosin S15 with a three-dimensional reconstruction from electron micrographs of frozen-hydrated F-actin decorated with recombinant Dictyostelium myosin S1. The accuracy of the reconstruction shows the position of actin and myosin molecules unambiguously.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Toyoshima, Y. Y. et al. Nature 328, 536–539 (1987).
Kishino, A. & Yanagida, T. Nature 334, 74–76 (1988).
Holmes, K. C., Popp, D., Gebhard, W. & Kabsch, W. Nature 347, 44–49 (1990).
Lorenz, M., Rypniewski, W. R., Popp, D. & Holmes K. C. J. molec. Biol. (in the press).
Rayment, I. et al. Science (in the press).
Manstein, D. J., Ruppel, K. M. & Spudich, J. A. Science 246, 656–658 (1989).
Schröder, R. R., Hofmann, W. & Menetret, J.-F. J. struct. Biol. 105, 28–34 (1990).
Moore, P. B., Huxley, H. E. & DeRosier, D. J. J. molec. Biol. 50, 279–295 (1970).
Jones, T. A. Meth. Enzym. 115, 157–171 (1985).
Rayment, I. et al. Science (in the press).
Onishi, H., Maita, T., Matsuda, G. & Fujiwara, K. J. biol. chem. 265, 19362–19368 (1990).
Maita, T. et al. J. Biochem., Tokyo 110, 75–87 (1991).
Brenner, B., Schoenberg, M., Chalovich, J. M., Greene, L. E. & Eisenberg, E. Proc. natn. Acad. Sci. U.S.A. 79, 7288–7291 (1982).
Geeves M. A. Phil. Trans. R. Soc. Lond. B336, 63–70 (1992).
Geisterfer-Lowrance, A. T. et al. Cell 62, 999–1006 (1990).
Brzeska, H., Lynch, T. J., Martin, B., Corigliano-Murphy, A. & Korn, E. D. J. biol. Chem. 265, 16138–16144 (1990).
Milligan, R. A. Whittaker, M. & Safer, D. Nature 348, 217–221 (1990).
Kron, S. J. & Spudich, J. A. Proc. natn. Acad. Sci. U.S.A. 83, 6272–6276 (1988).
Troung, T., Medley, Q. G. & Côté, G. P. J. biol. Chem. 267, 9767–9772 (1992).
Ritchie, M. D., Geeves, M.A., Woodward, S. K. A. & Manstein, D. J. Proc. natn. Acad. Sci. U.S.A. (in the press).
Spudich, J. A. & Watt, S. J. J. biol. Chem. 246, 4866–4871 (1971).
Smith, M. F. & Langmore, J. P. J. molec. Biol. 226, 763–774 (1992).
Schröder, R. R., Manstein, D., Jahn, W. & Spudich, J. A. Proc. 51st MSA Conf., Cincinnati (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schröder, R., Manstein, D., Jahn, W. et al. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature 364, 171–174 (1993). https://doi.org/10.1038/364171a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/364171a0
This article is cited by
-
The actomyosin interface contains an evolutionary conserved core and an ancillary interface involved in specificity
Nature Communications (2021)
-
Hypertrophic cardiomyopathy and the myosin mesa: viewing an old disease in a new light
Biophysical Reviews (2018)
-
The myosin mesa and the basis of hypercontractility caused by hypertrophic cardiomyopathy mutations
Nature Structural & Molecular Biology (2017)
-
Fitting \(\alpha \) α \(\beta \) β -crystalline structure onto electron microscopy based on SO(3) rotation group theory
Journal of Combinatorial Optimization (2015)
-
Moving into the cell: single-molecule studies of molecular motors in complex environments
Nature Reviews Molecular Cell Biology (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.