Abstract
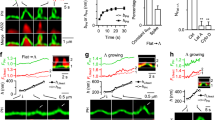
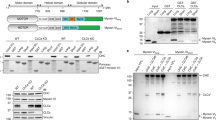
Do the coats on vesicles budded from the Golgi apparatus actually cause the budding, or do they simply coat buds (Fig. 1)? One view (the membrane-mediated budding hypothesis1) is that budding is an intrinsic property of Golgi membranes not requiring extrinsic coat proteins. Assembly of coats from dispersed subunits is superimposed upon the intrinsic budding process and is proposed to convert the tips of tubules into vesicles. The alternative view (the coat-mediated budding hypothesis1) is that coat formation provides the essential driving force for budding. The membrane-mediated budding hypothesis was inspired by the microtubule-dependent extension of apparently uncoated, 90-nm-diameter membrane tubules from the Golgi apparatus2 and other organelles3–5 in vivo after treatment with brefeldin A, a drug that inhibits the assembly of coat proteins onto Golgi membranes6–9. This hypothesis predicts that tubules will be extended when coat proteins are unavailable to convert tubule-derived membrane into vesicles. Here we use a cell-free system in which coated vesicles are formed from Golgi cisternae to show that, on the contrary, when budding diminishes as a result of immunodepletion of coat protein pools, tubules are not formed at the expense of vesicles. We conclude that coat proteins are required for budding from Golgi membranes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Klausner, R. D., Donaldson, J. G. & Lippincott-Schwartz, J. J. Cell Biol. 116, 1071–1080 (1992).
Lippincott-Schwartz, J. et al. Cell 60, 821–836 (1990).
Hunziker, W., Whitney, J. A. & Mellman, I. Cell 67, 617–628 (1991).
Lippincott-Schwartz, J. et al. Cell 67, 601–616 (1991).
Wood, S. A., Park, J. E. & Brown, W. J. Cell 67, 691–600 (1991).
Donaldson, J. G. et al. J. Cell Biol 111, 2295–2306 (1990).
Duden, R. et al. Cell 64, 649–665 (1991).
Orci, L. et al. Cell 64, 1183–1195 (1991).
Robinson, M. S. & Kreis, T. E. Cell 69, 129–138 (1992).
Balch, W. E. et al. Cell 39, 405–416 (1984)
Orci, L., Glick, B. S. & Rothman, J. E. Cell 46, 171–184 (1986).
Melancon, P. et al. Cell 51, 1053–1062 (1987).
Malhotra, V. et al. Cell 58, 329–336 (1989).
Serafini, T. et al. Nature 349, 215–220 (1991).
Waters, M. G., Serafini, T. & Rothman, J. E. Nature 349, 248–251 (1991).
Kahn, R. A. et al. J. biol Chem. 266, 2606–2614 (1991).
Serafini, T. et al. Cell 67, 239–253 (1991).
Donaldson, J. G. et al. Science 254, 1197–1199 (1991).
Donaldson, J. G. et al. Proc. natn. Acad. Sci. U.S.A. 89, 6408–6412 (1992).
Helms, J. B. & Rothman, J. E. Nature 360, 352–354 (1992).
Mellman, I. & Simons, K. Cell 68, 829–840 (1992).
Pelham, H. R. B. Cell 67, 449–451 (1991).
Pearse, B. M. F. & Bretscher, M. S. A. Rev Biochem 50, 85–101 (1981).
Moore, M. S., Mahaffey, D. T., Brodsky, F. M. & Anderson, R. G. W. Science 236, 558–563 (1987).
Smythe, E., Pypaert, M., Lucocq, J. & Warren, G. J. Cell Biol. 108, 843–853 (1989).
Orci, L. et al. Cell 56, 357–368 (1989).
Cluett E. B., Wood, S. A., Banta, M. & Brown, W. J. J. Cell Biol. 120, 15–24 (1993).
Allan, V. J. & Kreis, T. E. J. Cell Biol. 103, 2229–2239 (1986).
Waters, M. G., Beckers, C. J. M. & Rothman, J. E. Meth. Enzym. 219, 331 (1992).
Palmer, D. J., Orci, L. & Rothman, J. E. J. biol. Chem. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Orci, L., Palmer, D., Ravazzola, M. et al. Budding from Golgi membranes requires the coatomer complex of non-clathrin coat proteins. Nature 362, 648–652 (1993). https://doi.org/10.1038/362648a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/362648a0
This article is cited by
-
GORAB scaffolds COPI at the trans-Golgi for efficient enzyme recycling and correct protein glycosylation
Nature Communications (2019)
-
Rab1-dependent ER–Golgi transport dysfunction is a common pathogenic mechanism in SOD1, TDP-43 and FUS-associated ALS
Acta Neuropathologica (2015)
-
Components of the CtBP1/BARS-dependent fission machinery
Histochemistry and Cell Biology (2013)
-
ArfGAP1 generates an Arf1 gradient on continuous lipid membranes displaying flat and curved regions
The EMBO Journal (2010)
-
COPI-mediated Transport
Journal of Membrane Biology (2006)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.