Abstract
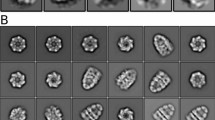
WE recently reported that a wide range of thermophilic archaebacteria have a novel ATPase complex which accumulates to high levels upon heat shock and may be a new type of molecular chaperone related to the chaperonins1. Striking similarities between the complex, referred to here as the 'thermosome', and the subsequently characterized chaperones TF55 of the thermophilic archaebacterium Sulfolobus shibatae2 and TCP-1 from eukaryotic cytosol2–5 suggest that these proteins belong to a single family. Here we determine the three-dimensional structure of the thermosome from Pyrodictium occultum by random conical tilt reconstruction from electron micrographs. The reconstruction reveals a complex consisting of two rings of eight subunits each, stacked face-to-face. The subunits are kidney-shaped and composed of at least two domains. In the centre of the thermosome is a large cavity of about 6.7 nm diameter; the opening of this cavity on each face of the complex is partially blocked by a mass which appears to be weakly connected to the eight-membered ring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Phipps, B. M., Hoffmann, A., Stetter, K. O. & Baumeister, W. EMBO J. 10, 1711–1722 (1991).
Trent, J. D., Nimmesgern, E., Wall, J. S., Hartl, F.-U. & Horwich, A. L. Nature 354, 490–493 (1991).
Gao, Y., Thomas, J. O., Chow, R. L., Lee, G.-H. & Cowan, N. J. Cell 69, 1043–1050 (1992).
Lewis, V. A., Hynes, G. M., Zheng, D., Saibil, H. & Willison, K. Nature 358, 249–252 (1992).
Yaffe, M. B. et al. Nature 358, 245–248 (1992).
Gething, M.-J. & Sambrook, J. Nature 355, 33–45 (1992).
Ellis, R. J. Nature 328, 378–379 (1987).
Goloubinoff, P., Christeller, J. T., Gatenby, A. A. & Lorimer, G. H. Nature 342, 884–889 (1989).
Hemmingsen, S. M. et al. Nature 333, 330–334 (1988).
Ellis, R. J. & van der Vies, S. M. A. Rev. Biochem. 60, 321–347 (1991).
Ellis, R. J. Nature 358, 191 (1992).
Trent, J. D., Osipiuk, J. & Pinkau, T. J. Bact. 172, 1478–1484 (1990).
Baumeister, W. et al. FEBS Lett. 241, 239–245 (1988).
Ellis, R. J. Science 250, 954–959 (1990).
Gupta, R. S. Biochem. Int. 4, 833–841 (1990).
Radermacher, M. J. Electron Microsc. Techn. 9, 359–394 (1988).
Zemlin, F. J. Electron Microsc. Techn. 11, 251–257 (1989).
Hutchinson, E. G., Tichelaar, W., Hofhaus, G., Weiss, H. & Leonard, K. R. EMBO J. 8, 1485–1490 (1989).
Viitanen, P. V. et al. J. biol. Chem. 267, 695–698 (1992).
Flaherty, K. M., DeLuca-Flaherty, C. & McKay, D. B. Nature 346, 623–628 (1990).
Rippmann, F., Taylor, W. R., Rothbard, J. B. & Green, N. M. EMBO J. 10, 1053–1059 (1991).
Carrascosa, J. L., Abella, G., Marco, S. & Carazo, J. M. J. struct. Biol. 104, 2–8 (1990).
Flynn, G. C., Pohl, J., Flocco, M. T. & Rothman, J. E. Nature 353, 726–730 (1991).
Martin, J. et al. Nature 352, 36–42 (1991).
Langer, T., Pfeifer, G., Martin, J., Baumeister, W. & Hartl, F.-U. EMBO J. 11, 4757–4765 (1992).
Creighton, T. E. Nature 352, 17–18 (1991).
Saibil, H., Dong, Z., Wood, S. & auf der Mauer, A. Nature 353, 25–26 (1991).
Viitanen, P. V. et al. Biochemistry 29, 5665–5671 (1990).
Hegerl, R. & Altbauer, A. Ultramicroscopy 9, 109–116 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Phipps, B., Typke, D., Hegerl, R. et al. Structure of a molecular chaperone from a thermophilic archaebacterium. Nature 361, 475–477 (1993). https://doi.org/10.1038/361475a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/361475a0
This article is cited by
-
Diversity in heat shock protein families: functional implications in virus infection with a comprehensive insight of their role in the HIV-1 life cycle
Cell Stress and Chaperones (2021)
-
Stress-tolerant non-conventional microbes enable next-generation chemical biosynthesis
Nature Chemical Biology (2020)
-
Stress response physiology of thermophiles
Archives of Microbiology (2017)
-
Characterization of CCTα and evaluating its expression in the mud crab Scylla paramamosain when challenged by low temperatures alone and in combination with high and low salinity
Cell Stress and Chaperones (2015)
-
Transcriptome analysis of heat stress response in switchgrass (Panicum virgatumL.)
BMC Plant Biology (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.