Abstract
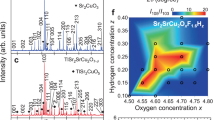
SUPERCONDUCTIVITY in the high-transition-temperature (high-Tc) copper oxide superconductors seems to arise from layers of copper–oxygen squares, pyramids or octahedra. Recently the compound Sr2CuO2CO3 was found to contain layers of CuO6 octahedra1 (Fig. 1), suggesting that it might be made superconducting by appropriate doping. But the presence of carbonate as an impurity is known to degrade the superconducting properties of materials such as and (refs 2, 3), much effort having been made to minimize residual carbon in these compounds by optimizing processing methods4. It is thus of some interest to see whether Sr2CuO2CO3 can be made superconducting despite the carbonate ions incorporated in the structure. Here we report the synthesis, at 50 atm oxygen partial pressure, of superconducting (0.4≤x≤0.65, y≈O.1), with Tc (onset) up to ∼40 K and zero resistance at up to ∼26K. The crystal structure contains CuO2 sheets alternating with , slabs, which serve as charge reservoir layers: substitution of ∼10% copper for carbon in the slabs introduces holes into the CuO2 sheets, making the compound superconducting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Miyazaki, Y. et al. Physica C191, 434–440 (1992).
Shaw, T. M., et al. J. Mater. Res. 5, 1176–1184 (1990).
Zandbergen, H. W., Cava, R. J., Krajewski, J. J. & Peck, Jr, W. F. Physica C179, 227–242 (1991).
Lindemer, T. B., Washburn, F. A., MacDougall, C. S. & Cavin, O. B. Physica C174, 135–143 (1991).
Lander, J. J. J. Am. chem. Soc. 73, 5794–5797 (1951).
Naka, S., Nakakita, F., Suwa, Y. & Inagaki, M. Bull. chem. Soc. Jpn 47, 1168–1171 (1974).
Izumi, F. et al. Physica C (in the press).
David, W. I. F. et al. Nature 327, 310–312 (1987).
Tokura, Y., Torrance, J. B., Huang, T. C. & Nazzal, A. I. Phys. Rev. B38, 7156–7159 (1988).
Tokura, Y. & Arima, T. Jpn J. appl. Phys. 29, 2388–2402 (1990).
Grande, V. B., Müller-Buschbaum, H. & Schweizer, M., Z. Anorg. allg. Chem. 428, 120–124 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kinoshita, K., Yamada, T. A new copper oxide superconductor containing carbon. Nature 357, 313–315 (1992). https://doi.org/10.1038/357313a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/357313a0
This article is cited by
-
Phase diagrams for the YBa2Cu3O7 family — ANNO 1996
Journal of Thermal Analysis (1997)
-
The formation of the Ba(CuOx)1−y(CO3)y
Journal of Thermal Analysis (1996)
-
A precursor route to prepare superconducting Sr2CuO2(CO3)1?x (BO3) x
Journal of Materials Science Letters (1996)
-
A new family of superconductors containing carbonate group
Journal of Superconductivity (1994)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.