Abstract
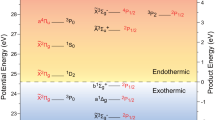
THE atmospheric sodium nightglow and luminescence of meteor trails, both of which occur at mesospheric altitudes of 85–95 km, are emitted by sodium atoms in the excited 2P state. The Chapman mechanism1–8 attributes these to the reaction between NaO and oxygen atoms, requiring an unusually high rate of formation of excited Na*(2P) relative to ground-state Na(2S) atoms from the NaO + O reaction. But laboratory studies of the kinetics9 show a very low Na*(2P) formation rate (branching ratio f< 0.01). NaO is itself formed by the reaction of sodium atoms with ozone. Molecular-beam experiments10 and photoelectron spectroscopy11 have shown recently that this reaction yields largely excited-state (2Σ+) NaO rather than the ground-state (2Π) species studied in the NaO + O kinetics experiments9, thereby suggesting a resolution of the apparent discrepancy with the Chapman mechanism. By extending the symmetry correlation between reactant and product electronic states considered by Bates and Ohja6, we show here that reaction of excited-state NaO with oxygen atoms does indeed yield branching ratios consistent with the Chapman mechanism. We infer that the NaO + O potential-energy surfaces leading to excited Na*(2P) atoms involve doublet rather than quartet spin configurations, and the branching ratio f is close to zero for ground-state NaO but ∼2/3 for excited-state NaO. If confirmed experimentally, this finding may enable the sodium nightglow to be used as a quantitative measure of mesospheric ozone concentrations5,7,8.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Chapman, S. Astrophys. J. 90, 309–316 (1939).
Hunten, D. M. in The Radiating Atmosphere (ed. McCormac, B. M.) 3–16 (Reidel, Dordrecht, 1971).
Kolb, C. E. & Elgin, J. B. Nature 263, 488–489 (1976).
Clemesha, B. R., Kirchhoff, V. W. J. H. & Simonich, D. M. J. geophys. Res. 83, 2499–2503 (1978).
Kirchhoff, V. W. J. H., Clemesha, B. R. & Simonich, D. M. J. geophys. Res. 84, 1323–1327 (1979).
Bates, D. R. & Ojha, P. C. Nature 286, 790–791 (1980).
Swider, W. J. geophys. Res. 91, 6742–6746 (1986).
Newman, A. L. J. geophys. Res. 93, 4067–4075 (1988).
Plane, J. M. C. & Husain, D. J. chem. Soc. Faraday Trans. II 82, 2047–2052 (1986).
Shi, X., Herschbach, D. R., Worsnop, D. R. & Kolb, C. E. J. phys. Chem. (in the press).
Dyke, J. M., Shaw, A. M. & Wright, T. G. in Gas-Phase Metal Reactions (ed. Fontijn, A.) (Elsevier, Amsterdam, in the press).
Ager, J. W. III, Talcott, C. L. & Howard, C. J. J. chem. Phys. 85, 5584–5592 (1986).
Worsnop, D. R., Zahniser, M. S. & Kolb, C. E. J. phys. Chem. 95, 3960–3964 (1991).
Gislason, E. A. in Alkali Vapors (eds Davidovits, P. & McFaddan, D. L.) 415–440 (Academic, New York, 1979).
Chikashi, Y., Masahara, F. & Hirota, E. J. chem. Phys. 90, 3033–3037 (1989).
Offerman, D., Friedrich, V., Ross, P. & von Zahn, U. Planet. Space Sci. 29, 747–764 (1981).
Sharp, W. E. Planet. Space Sci. 39, 617–626 (1991).
Herzberg, G. Electronic States of Polyatomic Molecules, 574–576 (Van Nostrand, Princeton, 1966).
Langhoff, S. R., Partridge, H. & Bauschlicher, C. W. Jr Chem. Phys. 153, 1–12 (1991).
Alexander, M. H. J. chem. Phys. 69, 3502–3517 (1978).
Fukui, K. Angew. Chem. int. Ed. Engl. 21, 801–809 (1982).
Herschbach, D. R. Angew. Chem. int. Ed. Engl. 26, 1221–1243 (1987).
Vaughan, G. Nature 296, 133–135 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Herschbach, D., Kolb, C., Worsnop, D. et al. Excitation mechanism of the mesospheric sodium nightglow. Nature 356, 414–416 (1992). https://doi.org/10.1038/356414a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/356414a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.