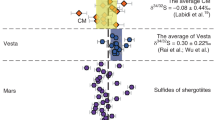
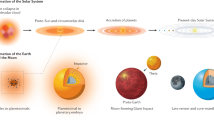
Abstract
THE planets and meteorites originated in a disk of gas and dust—the solar nebula—in which thermal processing fractionated the elements to varying degrees between gaseous and condensed phases1,2. A record of these effects is preserved in the chemical, mineralogical and isotopic compositions of meteorites, which accreted in the nebula. Previous attempts to interpret these fractionation effects have been based largely on thermodynamic calculations3,4, which assume that phases remained in equilibrium with one another. Vapour–solid equilibria in the system Mg–Si–O–H have also been studied experimentally5. But equilibrium is only a first approximation to the real situation: internal evidence in the chondritic meteorites6 shows that the thermal processing was rapid, and kinetic effects (non-equilibrium fractionation) must have played an important part in the evaporation and condensation events that created the chondrules, refractory inclusions and dust grains that are preserved in chondrites. Here I report the results of experiments to study the kinetics of evaporation of Mg2SiO4 (forsterite), the most abundant mineral in planets and meteorites. Solid and liquid Mg2SiO4 evaporate very slowly, only about one-tenth as fast as they would if the vapour species produced by evaporation were those predicted by equilibrium thermodynamics, and if no energy barrier in excess of the energy of reaction impeded the process. This large kinetic effect is due mainly to the transient production of gaseous SiO2 on evaporation. As a result, forsterite may evaporate at the same rate as an intrinsically more refractory material.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Urey, H. C. The Planets (Yale University Press, New Haven, 1952).
Anders, E. Phil. Trans. R. Soc. A285, 23–40 (1977).
Lord, H. C. Icarus 4, 279–288 (1965).
Grossman, L. Geochim. cosmochim. Acta 36, 597–619 (1972).
Mysen, B. O. & Kushiro, I. Am. Miner. 73, 1–19 (1988).
Wood, J. A. A. Rev. Earth planet Sci. 16, 53–72 (1988).
Hirth, J. P. & Pound, G. M. Condensation and Evaporation (Pergamon, Oxford, 1963).
Langmuir, I. Phys. Rev. 2, 329–342 (1913).
Somorjai, G. A. & Lester, J. E. Prog. Solid State Chem. 4, (ed. Reiss, H.) 1–53 (Pergamon, Oxford, 1967).
Sasamoto, T., Lee, H. L. & Sata, T. Yogyo-Kyokai-Shi 82, 603–610 (1974).
Firsova, L. P. & Nesmeyanov, An. N. Soviet J. phys. Chem. 34, 1279–1281 (1960).
Nagai, S., Niwa, K., Shinmei, M. & Yokokawa, T. JCS Faraday Trans.I 69, 1628–1634 (1973).
Chase, M. W. et al. JANAF Thermochemical Tables, 3rd edn (Dow Chemical Company, Midland, 1986).
Nagahara, R., Kushiro, I., Mysen, B. O. & Mori, H. Nature 331, 516–518 (1988).
Sasamoto, T., Kobayashi, M. & Sata, T. Shitsuryo Bunseki 29, 249–255 (1981).
Muenow, D. W., Uy, O. M. & Margrave, J. L. J. inorg. nucl. Chem. 32, 3459–3467 (1970).
Brittain, R. D., Lau, K. H. & Hildenbrand, D. L. J. phys. Chem. 86, 5072–5075 (1982).
Northrop, D. A. J. phys. Chem. 75, 118–132 (1971).
Motzfeldt, K. J. phys. Chem. 59, 139–147 (1955).
Porter, R. F., Chupka, W. A. & Inghram, M. G. J. chem. Phys. 23, 216–217 (1955).
Hashimoto, A., Holmberg, B. B. & Wood, J. A. Meteoritics 24, 276 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hashimoto, A. Evaporation kinetics of forsterite and implications for the early solar nebula. Nature 347, 53–55 (1990). https://doi.org/10.1038/347053a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/347053a0
This article is cited by
-
Specificity Switching Pathways in Thermal and Mass Evaporation of Multicomponent Hydrocarbon Droplets: A Mesoscopic Observation
Scientific Reports (2017)
-
Interdiffusion of Mg–Fe in olivine at 1,400–1,600 °C and 1 atm total pressure
Physics and Chemistry of Minerals (2013)
-
Solar asteroid diversion
Nature (1993)
-
Isotope mass fractionation during evaporation of Mg2Si04
Nature (1990)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.