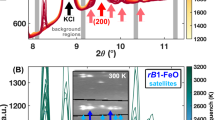
Abstract
Fe2+ is the most abundant iron species in magmas and in many slags. Its structural role in these liquids is poorly understood, largely because of the difficulty of studying melts at high temperatures by direct structural methods. Whether Fe2+ behaves as a network modifier, network former or free-ion complex has not been adequately resolved, yet this is fundamental to understanding the properties of silicate liquids. Also uncharacterized are the structural changes accompanying the melt-to-glass transition in Fe-bearing silicate melts. Here we report the results of a high-temperature synchrotron-based X-ray absorption study of Fe in silicate glasses and melts of compositions near Na2FeSi3O8 and K2FeSi3O8. The glasses were also analysed by 57Fe Mossbauer spectroscopy. We conclude that Fe2+ is a four-coordinated network former in these melt/glass systems and that little structural relaxation occurs at the iron site during the melt-to-glass transition. These results and structural data for Fe22+SiO4 melts1 suggest the possibility of a pressure-induced change from four- to six-coordination for Fe2+ in magmas in the Earth's upper mantle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Waseda, Y. & Toguri, J. M. Metall. Trans. 9B, 595–601 (1978).
Lytle, F. W. et al. Nucl. Instrum. Meth. 226, 542–548 (1984).
Waychunas, G. A., Brown, G. E. Jr & Apted, M. J. Phys. Chem. Miner. 13, 31–47 (1986).
Hazen, R. M. & Finger, L. W. Comparative Crystal Chemistry (Wiley, New York, 1986).
Eisenberger, P. & Brown, G. S. Solid St. Commun. 29, 481–484 (1979).
Simanek, E. & Sroubek, Z. Phys. Rev. 163, 275–279 (1967).
Dollase, W. A. Phys. Chem. Miner. 6, 295–304 (1980).
Waychunas, G. A., Apted, M. J. & Brown, G. E. Jr Phys. Chem. Miner. 10, 1–9 (1983).
Mysen, B. O., Virgo, D., Neumann, E.-R. & Seifert, F. A. Am. Miner. 70, 317–331 (1985).
Calas, G. & Petiau, J. in The Structure of Non-Crystalline Materials (eds Gaskell, P. H., Parker, J. M. & Davis, E. A.) 18–28 (Taylor and Francis, London, 1982).
Waff, H. S. Geophys. Res. Lett. 2, 193–196 (1975).
Waseda, Y. & Toguri, J. M. Metall. Trans. 8B, 563–568 (1977).
Yin, C. D., Okuno, M., Morikawa, H. & Marumo, F. J. non-cryst. Solids 55, 131–141 (1983).
Sharma, S. & Yoder, H. S. Yb. Carnegie Instn Wash. 78, 526–532 (1979).
Matsui, Y. & Kawamura, K. Nature 285, 648–649 (1980).
Dempsey, M., Kawamura, K. & Henderson, C. Prog. exp. Petrology 6, 57–59 (1985).
Teo, B. K. & Lee, P. A. J. Am. Chem Soc. 101, 2815–2831 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Waychunas, G., Brown, G., Ponader, C. et al. Evidence from X-ray absorption for network-forming Fe2+ in molten alkali silicates. Nature 332, 251–253 (1988). https://doi.org/10.1038/332251a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/332251a0
This article is cited by
-
Influence of Glass Structure on the Dissolution Behavior of Fe from Glassy Slag of CaO–SiO2–FeOx System
Journal of Sustainable Metallurgy (2021)
-
In situ pair distribution function analysis of crystallizing Fe-silicate melts
Journal of Materials Science (2021)
-
Effect of CaCl2 Addition on the Viscosity of CaO–SiO2–FeOx Steelmaking Slag System
Metallurgical and Materials Transactions B (2021)
-
Redox-structure dependence of molten iron oxides
Communications Materials (2020)
-
Structure and Viscosity of Molten CaO-SiO2-FexO Slag During the Early Period of Basic Oxygen Steelmaking
Metallurgical and Materials Transactions B (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.