Abstract
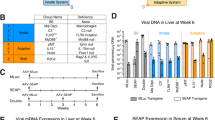
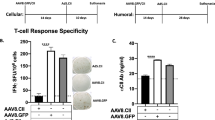
Neutralizing antibodies (nAB) at the time of administration hamper the effectiveness of adeno-associated virus (AAV) as a clinical DNA delivery system. The present study was designed to investigate if AAV re-administration in muscle tissue is dependent on the nAB titer. Recombinant (r)AAV serotype 1, as a promising candidate for targeting skeletal muscle, was used for gene delivery. C57Bl/6 mice were infected intramuscularly with doses between 1 × 109 and 5 × 1010 virus particles (vp) of AAV1-expressing luciferase (AAV1-luc) or human interferon-β (AAV1-hIFNβ). Increasing transgene expression was observed over the first 2 months and anti-AAV1 nAB titers peaked between weeks 4 and 8. Six months after the first administration, 5 × 1010 vp of AAV1-IFNβ were re-administered. Following re-administration, nAB titers increased but did not significantly affect transgene expression from the AAV vector that had been administered first. In contrast, hIFNβ expression originating from the second vector administration was significantly diminished and reflected the nAB titer present at the day of re-administration. The present study extends earlier observations that preexisting nAB affects AAV1 re-administration. The level of nAB is proportional to the virus dose used for the first injection and transgene expression following re-administration is dependent on preexisting nAB titer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Blackow NR, Hoggan MD, Rowe WP . Serologic evidence for human infection with adeno-associated viruses. J Natl Cancer Inst 1968; 40: 319–327.
Gao GP, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 2004; 78: 6381–6388.
Grimm D, Kay MA . From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther 2003; 3: 281–304.
Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM . Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci USA 2002; 99: 11854–11859.
Gao GP, Vandenberghe LH, Wilson JM . New recombinant serotypes of AAV vectors. Curr Gene Ther 2005; 5: 285–297.
Warrington Jr HH, Herzog RW . Treatment of human disease by adeno-associated viral gene transfer. Hum Genet 2006; 199: 571–603.
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJE et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune system. Nat Med 2006; 12: 342–347.
McPhee SWJ, Janson CG, Li C, Samulski RJ, Camp AS, Francis JD et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med 2006; 8: 577–588.
Jian H, Pierce GF, Ozelo MC, DePaula EV, Vargas JA, Smith P et al. Evidence of multilayer factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther 2006; 14: 452–455.
Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Gene Therapy 2003; 101: 2963–2972.
Erles K, Sebokova P, Schlehofer JR . Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol 1999; 59: 406–411.
Moskalenko M, Chen L, van Roey M, Donahue BA, Snyder RO, McArthur JG et al. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol 2000; 74: 1761–1766.
Chirmule N, Propert K, Magosim S, Qian Y, Qian R, Wilson J . Immune responses to adenovirus and adeno-associated virus in humans. Gene Therapy 1999; 6: 1574–1583.
Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 2003; 101: 2963–2972.
Moss RB, Rodman D, Spencer LT . Repeated adeno-associated virus serotype 2 aerosol-mediated cystis fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 2004; 125: 509–521.
Halbert CL, Standaert TA, Aitken ML, Alexander IE, Russell DW, Miller AD . Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol 1997; 71: 5932–5941.
Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P et al. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol 2000; 74: 2420–2425.
Xiao W, Chirmurle N, Schnell MA, Tazelaar J, Hughes JV, Wilson JM . Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors. Mol Ther 2000; 1: 323–329.
Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM . Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol 2001; 75: 6199–6203.
Ge Y, Powell S, vanRoey M, McArthur JG . Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood 2001; 97: 3733–3737.
Peden CS, Burger C, Muzyzka N, Mandel RJ . Circulating anti-wild-type adeno-associated virus type-2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated but not rAAV5-mediated gene transfer in the brain. J Virol 2004; 78: 6344–6359.
Zhang YC, Powers M, Wasserfall C, Brusko T, Song S, Flotte T et al. Immunity to adeno-associated virus serotype 2 delivered transgenes imparted by genetic predisposition to autoimmunity. Gene Therapy 2004; 11: 233–240.
Kok MR, Voutetakis A, Yamano S, Wang J, Cotrim A, Katano H et al. Immune responses following salivary gland administration of recombinant adeno-associated serotype 2 vectors. J Gene Med 2005; 7: 432–441.
Riviere C, Danos O, Douar AM . Long term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Therapy 2006; 13: 1300–1308.
Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing toters in SCID mice. Blood 2006; 107: 1810–1817.
Lo WD, Qu G, Sferra TJ, Clark R, Chen R, Johnson PR . Adeno-associated virus-mediated gene transfer to the brain: duration and modulation of expression. Hum Gene Ther 1999; 10: 201–213.
Jerebtsova M, Batshaw ML, Ye X . Humoral immune response to recombinant adenovirus and adeno-associated virus after in utero administration of viral vectors in mice. Pediatr Res 2002; 52: 95–104.
Limberis MP, Wilson JM . Adeno-associated virus serotype 9 vectors transduce murine alveolar nasal epithelia and can be readministered. Proc Natl Acad Sci USA 2006; 103: 12993–12998.
Mastakow MY, Baer K, Symes CW, Leichtlein CB, Kotin RM, During MJ . Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J Virol 2002; 76: 8446–8454.
Anand V, Chirmule N, Fersh M, Maguire AM, Bennett J . Additional transduction events after subretinal readministration of recombinant adeno-associated virus. Hum Gene Ther 2000; 11: 449–457.
Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM . Gene therapy vectors based on adeno-associated virus type 1. J Virol 1999; 70: 8098–8108.
Chao H, Liu Y, Rabimowitz J, Li C, Samulski RJ, Welsh CE . Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther 2000; 2: 619–623.
Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW et al. Long term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 2005; 105: 1424–1430.
Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet 2000; 24: 257–261.
Brockstedt DG, Podsakoff GM, Fong L, Kurtzman G, Mueller-Rucholtz W, Engleman EG . Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin Immunol 1999; 92: 67–75.
Szymanski P, Kretschmer PJ, Bauzon MM, Jin F, Qian HS, Rubanyi GM et al. Development and validation of a robust and versatile one-plasmid regulated gene expression system. Mol Ther 2007; 15: 1340–1347.
Auricchio A, Hildinger M, O'Connor E, Gao GP, Wilson JM . Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther 2001; 12: 71–76.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petry, H., Brooks, A., Orme, A. et al. Effect of viral dose on neutralizing antibody response and transgene expression after AAV1 vector re-administration in mice. Gene Ther 15, 54–60 (2008). https://doi.org/10.1038/sj.gt.3303037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3303037
Keywords
This article is cited by
-
AAV2-VEGF-B gene therapy failed to induce angiogenesis in ischemic porcine myocardium due to inflammatory responses
Gene Therapy (2022)
-
Tissue and cell-type-specific transduction using rAAV vectors in lung diseases
Journal of Molecular Medicine (2021)
-
Molekulare Therapien bei neuromuskulären Erkrankungen im Kindesalter – Große Hoffnungen und unbekannte Risiken
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2020)
-
Adeno-associated virus vector as a platform for gene therapy delivery
Nature Reviews Drug Discovery (2019)
-
Gentherapien für neuromuskuläre Erkrankungen
Der Nervenarzt (2019)