Abstract
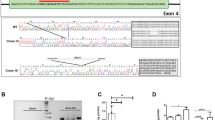
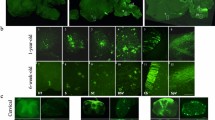
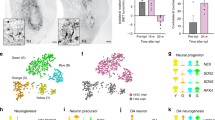
Pluripotency, virtually unlimited self-renewal and amenability to genetic modification make embryonic stem (ES) cells an attractive donor source for cell-mediated gene therapy. In this proof of concept study, we explore whether glial precursors derived from murine ES cells (ESGPs) and engineered to overexpress human arylsulfatase A (hASA) can cross-correct the metabolic defect in an animal model of metachromatic leukodystrophy (MLD). Transfected ES cells showed an up to 30-fold increase in ASA activity. Following in vitro differentiation, high expression of ASA was found in all stages of neural and glial differentiation. hASA-overexpressing ESGPs maintained their ability to differentiate into astrocytes and oligodendrocytes in vitro and in vivo. After transplantation into the brain of neonatal ASA-deficient mice, hASA-overexpressing ESGPs were found to incorporate into a variety of host brain regions. Four weeks after engraftment, immunofluorescence analyses with an antibody to sulfatide revealed a 46.7±4.0% reduction of immunoreactive sulfatide deposits in the vicinity of the hASA-positive engrafted cells, thereby significantly extending the rate of sulfatide reduction achieved by the endogenous ASA activity of non-hASA-transfected control cells (21.1±5.8%). These findings provide first in vivo evidence that ES cells may serve as a potential donor source for cell-mediated enzyme delivery in storage disorders such as MLD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
von Figura K, Gieselmann V, Jaeken J . Metachromatic leukodystrophy. In: Scriver CR et al. (eds). The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill: New York, 2001, pp 3695–3724.
Hess B, Saftig P, Hartmann D, Coenen R, Lullmann-Rauch R, Goebel HH et al. Phenotype of arylsulfatase A-deficient mice: relationship to human metachromatic leukodystrophy. Proc Natl Acad Sci USA 1996; 93: 14821–14826.
Peters C, Steward CG . Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant 2003; 31: 229–239.
Matzner U, Hartmann D, Lullmann-Rauch R, Coenen R, Rothert F, Mansson JE et al. Bone marrow stem cell-based gene transfer in a mouse model for metachromatic leukodystrophy: effects on visceral and nervous system disease manifestations. Gene Therapy 2002; 9: 53–63.
Biffi A, De Palma M, Quattrini A, Del Carro U, Amadio S, Visigalli I et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Invest 2004; 113: 1118–1129.
Molander-Melin M, Pernber Z, Franken S, Gieselmann V, Mansson JE, Fredman P . Accumulation of sulfatide in neuronal and glial cells of arylsulfatase A deficient mice. J Neurocytol 2004; 33: 417–427.
Consiglio A, Quattrini A, Martino S, Bensadoun JC, Dolcetta D, Trojani A et al. In vivo gene therapy of metachromatic leukodystrophy by lentiviral vectors: correction of neuropathology and protection against learning impairments in affected mice. Nat Med 2001; 7: 310–316.
Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC et al. Replacement therapy for inherited enzyme deficiency – macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med 1991; 324: 1464–1470.
Neufeld EF . Enzyme replacement therapy. In: Platt FM, Walkley SV (eds). Lysosomal Disorders of the Brain. Oxford University Press: Oxford, 2004, pp 327–338.
Matzner U, Herbst E, Hedayati KK, Lullmann-Rauch R, Wessig C, Schroder S et al. Enzyme replacement improves nervous system pathology and function in a mouse model for metachromatic leukodystrophy. Hum Mol Genet 2005; 14: 1139–1152.
Brüstle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science 1999; 285: 754–756.
Glaser T, Perez-Bouza A, Klein K, Brüstle O . Generation of purified oligodendrocyte progenitors from embryonic stem cells. FASEB J 2005; 19: 112–114 (E-pub 2004 Oct 2014).
Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci USA 2000; 97: 6126–6131.
Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS . Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 2005; 49: 385–396.
Perez-Bouza A, Glaser T, Brüstle O . ES cell-derived glial precursors contribute to remyelination in acutely demyelinated spinal cord lesions. Brain Pathol 2005; 15: 208–216.
Niwa H, Yamamura K, Miyazaki J . Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991; 108: 193–199.
Schierau A, Dietz F, Lange H, Schestag F, Parastar A, Gieselmann V . Interaction of arylsulfatase A with UDP-N-acetylglucosamine: lysosomal enzyme-N-acetylglucosamine-1-phosphotransferase. J Biol Chem 1999; 274: 3651–3658.
Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RDG . Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev 1996; 59: 89–102.
Gieselmann V . Metachromatic leukodystrophy: recent research developments. J Child Neurol 2003; 18: 591–594.
Givogri MI, Galbiati F, Fasano S, Amadio S, Perani L, Superchi D et al. Oligodendroglial progenitor cell therapy limits central neurological deficits in mice with metachromatic leukodystrophy. J Neurosci 2006; 26: 3109–3119.
Robinson AJ, Crawley AC, Hopwood JJ . Over-expression of human lysosomal alpha-mannosidase in mouse embryonic stem cells. Mol Genet Metab 2005; 85: 203–212.
Snyder EY, Taylor RM, Wolfe JH . Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature 1995; 374: 367–370.
Lacorazza HD, Flax JD, Snyder EY, Jendoubi M . Expression of human beta-hexosaminidase alpha-subunit gene (the gene defect of Tay–Sachs disease) in mouse brains upon engraftment of transduced progenitor cells. Nat Med 1996; 2: 424–429.
Fredman P, Mattsson L, Andersson K, Davidsson P, Ishizuka I, Jeansson S et al. Characterization of the binding epitope of a monoclonal antibody to sulphatide. Biochem J 1988; 251: 17–22.
Ishizuka I . Chemistry and functional distribution of sulfoglycolipids. Prog Lipid Res 1997; 36: 245–319.
Lullmann-Rauch R, Matzner U, Franken S, Hartmann D, Gieselmann V . Lysosomal sulfoglycolipid storage in the kidneys of mice deficient for arylsulfatase A (ASA) and of double-knockout mice deficient for ASA and galactosylceramide synthase. Histochem Cell Biol 2001; 116: 161–169.
D'Hooge R, Coenen R, Gieselmann V, Lullmann-Rauch R, De Deyn PP . Decline in brainstem auditory-evoked potentials coincides with loss of spiral ganglion cells in arylsulfatase A-deficient mice. Brain Res 1999; 847: 352–356.
McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 1999; 5: 1410–1412.
Erdo F, Buhrle C, Blunk J, Hoehn M, Xia Y, Fleischmann B et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab 2003; 23: 780–785.
Dihne M, Bernreuther C, Hagel C, Wesche KO, Schachner M . Embryonic stem cell-derived neuronally committed precursor cells with reduced teratoma formation after transplantation into the lesioned adult mouse brain. Stem Cells 2006: doi:10.1634/stemcells.2005–0413.
Shihabuddin LS, Numan S, Huff MR, Dodge JC, Clarke J, Macauley SL et al. Intracerebral transplantation of adult mouse neural progenitor cells into the Niemann–Pick-A mouse leads to a marked decrease in lysosomal storage pathology. J Neurosci 2004; 24: 10642–10651.
Barton NW, Furbish FS, Murray GJ, Garfield M, Brady RO . Therapeutic response to intravenous infusions of glucocerebrosidase in a patient with Gaucher disease. Proc Natl Acad Sci USA 1990; 87: 1913–1916.
Sakurai K, Iizuka S, Shen JS, Meng XL, Mori T, Umezawa A et al. Brain transplantation of genetically modified bone marrow stromal cells corrects CNS pathology and cognitive function in MPS VII mice. Gene Therapy 2004; 11: 1475–1481.
Matzner U, Habetha M, Gieselmann V . Retrovirally expressed human arylsulfatase A corrects the metabolic defect of arylsulfatase A-deficient mouse cells. Gene Therapy 2000; 7: 805–812.
Stein C, Gieselmann V, Kreysing J, Schmidt B, Pohlmann R, Waheed A et al. Cloning and expression of human arylsulfatase A. J Biol Chem 1989; 264: 1252–1259.
Swiatek PJ, Gridley T . Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev 1993; 7: 2071–2284.
Tucker KL, Wang Y, Dausman J, Jaenisch R . A transgenic mouse strain expressing four drug-selectable marker genes. Nucleic Acids Res 1997; 25: 3745–3746.
Schmandt T, Glaser T, Brüstle O . Lineage selection and transplantation of ES cell-derived neural precursors. In: Notarianni E, Evans M (eds). Embryonic Stem Cells – A Practical Approach. Oxford University Press: Oxford, 2006, pp 189–217.
Baum H, Dodgson KS, Spencer B . The assay of arylsulphatases A and B in human urine. Clin Chim Acta 1959; 4: 453–455.
Franceschini IA, Feigenbaum-Lacombe V, Casanova P, Lopez-Lastra M, Darlix JL, Dalcq MD . Efficient gene transfer in mouse neural precursors with a bicistronic retroviral vector. J Neurosci Res 2001; 65: 208–219.
Lopez-Lastra M, Gabus C, Darlix JL . Characterization of an internal ribosomal entry segment within the 5′ leader of avian reticuloendotheliosis virus type A RNA and development of novel MLV-REV-based retroviral vectors. Hum Gene Ther 1997; 8: 1855–1865.
Pernber Z, Molander-Melin M, Berthold CH, Hansson E, Fredman P . Expression of the myelin and oligodendrocyte progenitor marker sulfatide in neurons and astrocytes of adult rat brain. J Neurosci Res 2002; 69: 86–93.
Molander M, Berthold CH, Persson H, Fredman P . Immunostaining of ganglioside GD1b, GD3 and GM1 in rat cerebellum: cellular layer and cell type specific associations. J Neurosci Res 2000; 60: 531–542.
Acknowledgements
This work was supported by grants from the BMBF (01GN0108 and 01GN0502), the Hertie Foundation and the Alexander von Humboldt Foundation (research fellowship grant for AP-B). We would like to acknowledge the excellent technical assistance of Rachel Konang, Ivonne Becker and Clara Alfaro Cervelló. We thank Rolf Fimmers for the help in statistical analysis. The pREV-PLAP and pREV-ECFP retroviral vectors were kindly provided by Jean-Luc Darlix and Isabelle Franceschini.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klein, D., Schmandt, T., Muth-Köhne, E. et al. Embryonic stem cell-based reduction of central nervous system sulfatide storage in an animal model of metachromatic leukodystrophy. Gene Ther 13, 1686–1695 (2006). https://doi.org/10.1038/sj.gt.3302834
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302834
Keywords
This article is cited by
-
Sphingolipid lysosomal storage diseases: from bench to bedside
Lipids in Health and Disease (2021)
-
Arylsulfatase A Overexpressing Human iPSC-derived Neural Cells Reduce CNS Sulfatide Storage in a Mouse Model of Metachromatic Leukodystrophy
Molecular Therapy (2015)
-
Pathology and Current Treatment of Neurodegenerative Sphingolipidoses
NeuroMolecular Medicine (2010)
-
Lysosulfatide Regulates the Motility of a Neural Precursor Cell Line Via Calcium-mediated Process Collapse
Neurochemical Research (2009)
-
The Role and Metabolism of Sulfatide in the Nervous System
Molecular Neurobiology (2008)