Abstract
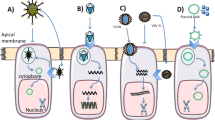
Our first review on progress and prospects in cystic fibrosis (CF) gene therapy was published in this series in October 2002. We now summarize the progress made since then and comment on the prospects for CF gene therapy over the next couple of years. Three clinical trials have been carried out, further supporting the proof-of-principle that gene transfer to the airway epithelium is feasible. Developments in viral and non-viral vectors, as well as recent alternative strategies such as gene repair, trans-splicing and stem cell therapy will be reviewed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Virella-Lowell I, Zusman B, Foust K, Loiler S, Conlon T, Song S et al. Enhancing rAAV vector expression in the lung. J Gene Med 2005; 7: 842–850.
Sirninger J, Muller C, Braag S, Tang Q, Yue H, Detrisac C et al. Functional characterization of a recombinant adeno-associated virus 5-pseudotyped cystic fibrosis transmembrane conductance regulator vector. Hum Gene Ther 2004; 15: 832–841.
Gao G, Vandenberghe LH, Wilson JM . New recombinant serotypes of AAV vectors. Curr Gene Ther 2005; 5: 285–297.
Yan Z, Zak R, Zhang Y, Ding W, Godwin S, Munson K et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol 2004; 78: 2863–2874.
Fischer AC, Beck SE, Smith CI, Laube BL, Askin FB, Guggino SE et al. Successful transgene expression with serial doses of aerosolized rAAV2 vectors in rhesus macaques. Mol Ther 2003; 8: 918–926.
Halbert C, Liggit D, Miller AD . Biosafety study of an adeno-associated virus 6 vector in rats. Pediatr Pulmonol 2005; S28: 277–278.
Lai Y, Yue Y, Liu M, Ghosh A, Engelhardt JF, Chamberlain JS et al. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat Biotechnol 2005; 11: 1435–1439.
Flotte TR, Zeitlin PL, Reynolds TC, Heald AE, Pedersen P, Beck S et al. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: a two-part clinical study. Hum Gene Ther 2003; 14: 1079–1088.
Gerard CJ, Dell'Aringa J, Hale KA, Klump WM . A sensitive, real-time, RNA-specific PCR method for the detection of recombinant AAV-CFTR vector expression. Gene Therapy 2003; 10: 1744–1753.
Flotte TR, Schwiebert EM, Zeitlin PL, Carter BJ, Guggino WB . Correlation between DNA transfer and cystic fibrosis airway epithelial cell correction after recombinant adeno-associated virus serotype 2 gene therapy. Hum Gene Ther 2005; 16: 921–928.
Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 2004; 125: 509–521.
Croteau GA, Martin DB, Camp J, Yost M, Conrad C, Zeitlin PL et al. Evaluation of exposure and health care worker response to nebulized administration of tgAAVCF to patients with cystic fibrosis. Ann Occup Hyg 2004; 48: 673–681.
Koehler DR, Sajjan U, Chow YH, Martin B, Kent G, Tanswell AK et al. Protection of Cftr knockout mice from acute lung infection by a helper-dependent adenoviral vector expressing Cftr in airway epithelia. Proc Natl Acad Sci USA 2003; 100: 15364–15369.
van Heeckeren AM, Scaria A, Schluchter MD, Ferkol TW, Wadsworth S, Davis PB . Delivery of CFTR by adenoviral vector to cystic fibrosis mouse lung in a model of chronic Pseudomonas aeruginosa lung infection. Am J Physiol Lung Cell Mol Physiol 2004; 286: L717–L726.
Hiatt P, Brunetti-Pierri N, Koehler D, McConnell R, Katkin J, Palmer D et al. Aerosol delivery of helper-dependent adenoviral vector into nonhuman primate lungs results in high efficiency pulmonary transduction with minimal toxicity. Mol Ther 2005; 11: 815.
Tosi MF, van Heeckeren A, Ferkol TW, Askew D, Harding CV, Kaplan JM . Effect of Pseudomonas-induced chronic lung inflammation on specific cytotoxic T-cell responses to adenoviral vectors in mice. Gene Therapy 2004; 11: 1427–1433.
Ferrari S, Geddes DM, Alton EW . Barriers to and new approaches for gene therapy and gene delivery in cystic fibrosis. Adv Drug Deliv Rev 2002; 54: 1373–1393.
Griesenbach U, Inoue M, Chan M, Farley R, Munkonge F, Wasowicz M et al. Characterisation of Sendai virus carrying a GFP-CFTR fusion protein in vitro and in vivo. Pediatr Pulmonol 2005; S28: 269.
Griesenbach U, McLachlan G, Somerton L, Collie D, Inoue M, Hironaka T et al. Repeated administration of Sendai virus into lung. Mol Ther 2005; 11: S327.
Griesenbach U, Boyton RJ, Somerton L, Garcia SE, Ferrari S, Owaki T et al. Effect of tolerance induction to immunodominant T-cell epitopes of Sendai virus on gene expression following repeat administration to lung. Gene Therapy 2006; 13: 449–456.
Medina MF, Kobinger GP, Rux J, Gasmi M, Looney DJ, Bates P et al. Lentiviral vectors pseudotyped with minimal filovirus envelopes increased gene transfer in murine lung. Mol Ther 2003; 8: 777–789.
Sinn PL, Shah AJ, Donovan MD, McCray Jr PB . Viscoelastic gel formulations enhance airway epithelial gene transfer with viral vectors. Am J Respir Cell Mol Biol 2005; 32: 404–410.
Mitomo K, Inoue M, Ueda Y, Ikegami M, Griesenbach U, Alton EWFW et al. Towards cystic fibrosis and airway gene therapy: evaluation of EGFP gene transfer in murine nasal airways mediated by simian immunodeficiency virus vectors pseudotyped with Sendai virus glycoproteins F and HN. Mol Ther 2005; 11: S139.
Desigaux L, Gourden C, Bello-Roufai M, Richard P, Oudrhiri N, Lehn P et al. Nonionic amphiphilic block copolymers promote gene transfer to the lung. Hum Gene Ther 2005; 16: 821–829.
Kichler A, Leborgne C, Marz J, Danos O, Bechinger B . Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc Natl Acad Sci USA 2003; 100: 1564–1568.
Meng QH, Robinson D, Jenkins RG, McAnulty RJ, Hart SL . Efficient transfection of non-proliferating human airway epithelial cells with a synthetic vector system. J Gene Med 2004; 6: 210–221.
Montier T, Delepine P, Marianowski R, Le Ny K, Le Bris M, Gillet D et al. CFTR transgene expression in primary DeltaF508 epithelial cell cultures from human nasal polyps following gene transfer with cationic phosphonolipids. Mol Biotechnol 2004; 26: 193–206.
Stern M, Ulrich K, Geddes DM, Alton EW . Poly (D, L-lactide-co-glycolide)/DNA microspheres to facilitate prolonged transgene expression in airway epithelium in vitro, ex vivo and in vivo. Gene Therapy 2003; 10: 1282–1288.
Griesenbach U, Wasowicz MY, Smith SN, Wodehouse T, Fox E, Somerton L et al. Endpoint assay development and validation of non-viral gene transfer agents in CF mice. Pediatr Pulmunol 2005; S28: 270.
McLachlan G, Davidson H, Fox E, Gill DR, Hyde SC, Griesenbach U et al. Ovine lung comparison of non-viral gene transfer agents for CF gene therapy. Pediatr Pulmunol 2005; S28: 278.
Pringle IA, Davies LA, McLachlan G, Collie DD, Gill DR, Hyde SC . Duration of reporter gene expression from naked pDNA in the mouse lung following direct electroporation and development of wire electrodes for sheep lung electroporation studies. Mol Ther 2004; 9: S56.
Xenariou S, Griesenbach U, Ferrari S, Dean P, Scheule R, Chend S et al. Magnetofection to enhance airway gene transfer. Mol Ther 2004; 9: S180.
Xenariou S, Liang H, Griesenbach U, Farley R, Somerton L, Singh C et al. Sonoporation increases non-viral gene transfer to the murine lung. Mol Ther 2005; 11: S139.
Bastonero S, Gargouri M, Ortiou S, Gueant JL, Merten MD . Inhibition by TNF-alpha and IL-4 of cationic lipid mediated gene transfer in cystic fibrosis tracheal gland cells. J Gene Med 2005; 7: 1439–1449.
Kim J, Chen CP, Rice KG . The proteasome metabolizes peptide-mediated nonviral gene delivery systems. Gene Therapy 2005; 12: 1581–1590.
Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther 2004; 15: 1255–1269.
De Semir D, Nadal M, Gonzalez JR, Larriba S, Avinyo A, Nunes V et al. Suitability of oligonucleotide-mediated cystic fibrosis gene repair in airway epithelial cells. J Gene Med 2003; 5: 625–639.
Liu X, Luo M, Zhang LN, Yan Z, Zak R, Ding W et al. Spliceosome-mediated RNA trans-splicing with recombinant adeno-associated virus partially restores cystic fibrosis transmembrane conductance regulator function to polarized human cystic fibrosis airway epithelial cells. Hum Gene Ther 2005; 16: 1116–1123.
Peebles D, Gregory LG, David A, Themis M, Waddington SN, Knapton HJ et al. Widespread and efficient marker gene expression in the airway epithelia of fetal sheep after minimally invasive tracheal application of recombinant adenovirus in utero. Gene Therapy 2004; 11: 70–78.
Cohen JC, Larson JE . Pathophysiologic consequences following inhibition of a CFTR-dependent developmental cascade in the lung. BMC Dev Biol 2005; 5: 2.
Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001; 105: 369–377.
Kotton DN, Fabian AJ, Mulligan RC . Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005; 33: 328–334.
Macpherson H, Keir P, Webb S, Samuel K, Boyle S, Bickmore W et al. Bone marrow-derived SP cells can contribute to the respiratory tract of mice in vivo. J Cell Sci 2005; 118: 2441–2450.
Yew NS, Wang KX, Przybylska M, Bagley RG, Stedman M, Marshall J et al. Contribution of plasmid DNA to inflammation in the lung after administration of cationic lipid:pDNA complexes. Hum Gene Ther 1999; 10: 223–234.
Acknowledgements
We thank all members of the UK CF Gene Therapy Consortium Strategy Group (Chris Boyd, Jane Davies, Deborah Gill, Steve Hyde and David Porteous) for critical reading and thank Lucinda Somerton for help with preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Griesenbach, U., Geddes, D., Alton, E. et al. Gene therapy progress and prospects: cystic fibrosis. Gene Ther 13, 1061–1067 (2006). https://doi.org/10.1038/sj.gt.3302809
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302809
Keywords
This article is cited by
-
Modulation of Treg function improves adenovirus vector-mediated gene expression in the airway
Gene Therapy (2014)
-
Longitudinal Cystic Fibrosis Care
Clinical Pharmacology & Therapeutics (2013)
-
Lysophosphatidylcholine as an adjuvant for lentiviral vector mediated gene transfer to airway epithelium: effect of acyl chain length
Respiratory Research (2010)
-
Toward Gene Therapy for Cystic Fibrosis Using a Lentivirus Pseudotyped With Sendai Virus Envelopes
Molecular Therapy (2010)
-
Progress and Prospects: The design and production of plasmid vectors
Gene Therapy (2009)