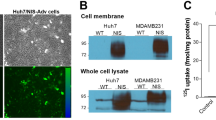
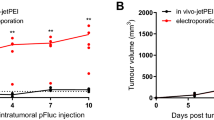
Abstract
Efficient gene delivery is a critical obstacle for gene therapy that must be overcome. Until current limits of gene delivery technology are solved, identification of systems with bystander effects is highly desirable. As an anticancer agent, radioactive iodine 131I has minimal toxicity. The physical characteristics of 131I decay allow radiation penetration within a local area causing bystander killing of adjacent cells. Accumulation of 131I mediated by the sodium iodide symporter (NIS) provides a highly effective treatment for well-differentiated thyroid carcinoma. Other types of cancer could also be treated by NIS-mediated concentration of lethal 131I radiation in tumor cells. Our group and others previously reported that a significant antitumor effect in mice was achieved after adenoviral delivery of rat or human NIS gene following administration of 3 mCi of 131I. We have also demonstrated 5–6-fold greater uptake of 125I by rat NIS over human NIS in human cancer cells. Recently, we reported the capability of the rat NIS and 131I to effectively induce growth arrest of relatively large tumors (approximately 800 mm3) in an animal model. In the present work tumor growth inhibition was achieved using adenoviral delivery of the rat NIS gene and 1 mCi of 131I (one-third of the dose used in earlier reports). We also demonstrated that a higher concentration of 123I was accumulated in the NIS-expressing tumors than in the thyroid 20 min after radioiodine administration. The highest intratumoral radioiodine concentration was observed along the needle track; however, the rat NIS-131I effectively induced growth arrest of tumor xenografts in mice through its radiological bystander effect. Importantly, the rat NIS allowed reducing the injected radioiodine dose by 70% with the same antitumor efficacy in pre-established tumors. These results suggest that the rat NIS gene may be advantageous compared to the human gene in its ability to enhance intratumoral 131I uptake.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Boland A, Ricard M, Opolon P, Bidart JM, Yeh P, Filetti S . Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res 2000; 60: 3484–3492.
Cho J-Y . A transporter gene (Sodium Iodide Symporter) for dual purposes in gene therapy: imaging and therapy. Curr Gene Ther 2002; 2: 393–402.
Grigsby PW, Siegel BA, Baker S, Eichling JO . Radiation exposure from outpatient radioactive iodine (131I) therapy for thyroid carcinoma. JAMA 2000; 283: 2272–2274.
Lin JD, Chao TC, Huang MJ, Weng HF, Tzen KY . Use of radioactive iodine for thyroid remnant ablation in well-differentiated thyroid carcinoma to replace thyroid reoperation. Am J Clin Oncol 1998; 21: 77–81.
Nagahara K, Yoza T, Naito Y, Konishi J, Fujita N . Evaluation of prophylactic postoperative 131I therapy for extended differentiated carcinoma of the thyroid gland. Auris Nasus Larynx 1985; 12 (Suppl 2): S67–S71.
Wartofsky L, Sherman SI, Gopal J, Slumberger M, Hay ID . The use of radioactive iodine in patients with papillary and follicular cancer. J Clin Endocrinol Metab 1998; 83: 4195–4203.
Levy O, Dai G, Riedel C, Ginter CS, Paul EM, Lebowitz AN et al. Characterization of the thyroid Na+/I− symporter with an anti-COOH terminus antibody. Proc Natl Acad Sci USA 1997; 94: 5568–5573.
De La Vieja A, Dohan O, Levy O, Carrasco N . Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev 2000; 80: 1083–1105.
Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M et al. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 2003; 24: 48–77.
Dai G, Levy O, Carrasco N . Cloning and characterization of the thyroid iodide transporter. Nature 1996; 379: 458–460.
Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL et al. Cloning of the human sodium iodide symporter. Biochem Biophys Res Commun 1996; 226: 339–345.
Mandell RB, Mandell LZ, Link CJ . Radioisotope concentrator gene therapy using the sodium iodide symporter gene. Cancer Res 1999; 59: 661–668.
Spitzweg C, Zhang S, Bergert ER, Castro MR, McIver B, Heufelder AE et al. Prostate-specific antigen (PSA) promoter driven androgen-inducible expression of sodium iodide symporter in prostate cancer cell lines. Cancer Res 1999; 59: 2136–2141.
Cho JY, Xing S, Liu X, Buckwalter TL, Hwa L, Sferra TJ et al. Expression and activity of human Na+/I− symporter in human glioma cells by adenovirus-mediated gene delivery. Gene Therapy 2000; 7: 740–749.
Spitzweg C, Dietz AB, O'Connor MK, Bergert ER, Tindall DJ, Young CY et al. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Therapy 2001; 8: 1524–1531.
Mitrofanova E, Unfer R, Vahanian N, Daniels W, Roberson E, Seregina T et al. Rat Sodium Iodide Symporter (rNIS) for radioiodide therapy of cancer. Clin Cancer Res 2004; 10: 6969–6976.
Faivre J, Clerc J, Gerolami R, Herve J, Longuet M, Liu B et al. Long-term radioiodine retention and regression of liver cancer after sodium iodide symporter gene transfer in wistar rats. Cancer Res 2004; 64: 8045–8051.
Groot-Wassink T, Aboagye EO, Wang Y, Lemoine NR, Reader AJ, Vassaux G . Quantitative imaging of Na/I symporter transgene expression using positron emission tomography in the living animal. Mol Ther 2004; 9: 436–442.
Heltemes LM, Hagan CR, Mitrofanova EE, Panchal RG, Guo J, Link CJ . The rat sodium iodide symporter gene permits more effective radioisotope concentration than human gene in human and rodent cancer cells. Cancer Gene Ther 2003; 10: 14–22.
Mitrofanova E, Unfer R, Vahanian N, Kane S, Carvour M, Link C . Effective growth arrest of a human colon cancer in mice using rNIS and radioiodine therapy. Human Gene Therapy 2005; 16: 1333–1337.
Mitrofanova E, Hagan C, Qi J, Seregina T, Link CJ . Sodium iodide symporter/radioactive iodine system has more efficient antitumor effect in three-dimensional structure. Anticancer Res 2003; 23: 2397–2404.
Dingli D, Diaz RM, Bergert ER, O'Connor MK, Morris JC, Russell SJ . Genetically targeted radiotherapy for multiple myeloma. Blood 2003; 102: 489–496.
Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, Zhao QH et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med 2000; 6: 871–878.
Kotani T, Ogata Y, Yamamoto I, Aratake Y, Kawano JI, Suganuma T et al. Characterization of gastric Na+/I− Symporter of the rat. Clin Immunol Immunopath 1998; 89: 271–278.
Gaut AW, Niu G, Krager KJ, Graham MM, Trask DK, Domann FE . Genetically targeted radiotherapy of head and neck squamous cell carcinoma using the sodium-iodide symporter (NIS). Head Neck 2004; 26: 265–271.
Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol 2002; 20: 557–566.
Valicenti RK, Bissonette EA, Chen C, Theodorescu D . Longitudinal comparison of sexual function after 3-dimensional conformal radiation therapy or prostate brachytherapy. J Urol 2002; 168: 2499–2504.
Acknowledgements
We are grateful to Dawn Bertrand and Stephen Kuhn for technical assistance. This research was supported by a Research Project Grant #RPG-98-091-01-MBC from the American Cancer Society and Grant #PC010633 from the Department of Defense, US Army.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitrofanova, E., Unfer, R., Vahanian, N. et al. Rat sodium iodide symporter allows using lower dose of 131I for cancer therapy. Gene Ther 13, 1052–1056 (2006). https://doi.org/10.1038/sj.gt.3302758
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302758
Keywords
This article is cited by
-
Virotheranostics, a double-barreled viral gun pointed toward cancer; ready to shoot?
Cancer Cell International (2020)
-
Sodium/iodide symporter gene transfection increases radionuclide uptake in human cisplatin-resistant lung cancer cells
Clinical and Translational Oncology (2015)