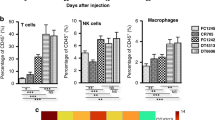
Abstract
MIG (monokine induced by interferon-γ) is a CXC chemokine ligand (CXCL9) that can potently inhibit angiogenesis, and displays thymus-dependent antitumor effects. The effectiveness of a treatment combining gene therapy with plasmid-borne MIG (pORF-MIG) and low-dose cisplatin chemotherapy was determined using colon carcinoma (CT26) and Lewis lung carcinoma (LL/2c) murine models. The program was carried out via intramuscular delivery of pORF-MIG at 100 μg/mouse twice a week for 4 weeks, and/or intraperitoneal delivery of cisplatin at 0.6 mg/kg/mouse every 3 days for 48 days. Tumor volume and survival time were evaluated after treatment. CD31 immunohistochemical staining in tumor tissues and alginate capsule models in vivo was used to evaluate angiogenesis. Induction of apoptosis and cytotoxic T-lymphocyte (CTL) activity were also assessed. The combination of pORF-MIG and low-dose cisplatin produced significant antitumor activity, with complete tumor regression in 4/10 of CT26 colon carcinomas and 3/10 of LL/2c lung carcinomas, low vascularity, in alginate capsules, apparently degraded tumor microvessel density, and increased induction of apoptotic and CTL activities compared with either treatment alone. This study suggests that the combination of pORF-MIG plus cisplatin augments the inhibition of angiogenesis and the induction of apoptosis or CTL activity, all of which enhance antitumor activity. These findings may prove useful in further explorations of the application of combinatorial approaches to the treatment of solid tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kerbel RS, Klement G, Pritchard KI, Kamen B . Continuous low-dose anti-angiogenic/metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol 2002; 13: 12–15.
Kamen BA, Rubin E, Aisner J, Glatstein E . High-time chemotherapy or high time for low dose. J Clin Oncol 2000; 18: 2935–2937.
Gasparini G . Metronomic scheduling: the future of chemotherapy? Lancet Oncol 2001; 2: 733–740.
Boehm T, Folkman J, Browder T, O'Reilly MS . Anti-angiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997; 390: 404–407.
Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 2000; 105: R15–R24.
Bocci G, Nicolaou KC, Kerbel RS . Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res 2002; 62: 6938–6943.
Kerbel RS . Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. BioEssays 1991; 13: 31–36.
Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 2000; 60: 1878–1886.
Folkman J . Clinical applications of research on angiogenesis. N Engl J Med 1995; 333: 1757–1763.
Harris AL . Anti-angiogenesis therapy and strategies for integrating it with adjuvant therapy. Recent Res Cancer Res 1998; 152: 341–352.
Eckhardt SG, Pluda JM . Development of angiogenesis inhibitors for cancer therapy. Invest New Drug 1997; 15: 1–3.
Folkman J . Angiogenesis inhibitors: a new class of drugs. Cancer Biol Ther 2003; 2: S127–S133.
Tandle A, Blazer DG, Libutti SK . Antiangiogenic gene therapy of cancer: recent developments. J Transl Med 2004; 2: 22.
Rollins BJ . Chemokines. Blood 1997; 90: 909–928.
Rossi D, Zlotnik A . The biology of chemokines and their receptors. Annu Rev Immunol 2000; 18: 217–242.
Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 1995; 270: 27348–27357.
Sgadari C, Farber JM, Angiolillo AL, Liao F, Teruya-Feldstein J, Burd PR et al. Mig, the monokine induced by interferon-γ, promotes tumor necrosis in vivo. Blood 1997; 89: 2635–2643.
Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H et al. Gene expression and production of the monokine induced by IFN-γ (MIG), IFN-inducible T cell-chemoattractant (I-TAC), and IFN-γ inducible protein-10(IP-10) chemokines by human neutrophils. J Immunol 1999; 162: 4928–4937.
Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM . Human Mig chemokine: biochemical and functional characterization. J Exp Med 1995; 182: 1301–1314.
Marx J . Angiogenesis: a boost for tumor starvation. Science 2003; 301: 452–454.
Kisker O, Becker CM, Prox D, Fannon M, D'Amato R, Flynn E et al. Continuous administration of endostatin by intraperitoneally implanted osmotic pump improves the efficacy and potency of therapy in a mouse xenograft tumor model. Cancer Res 2001; 61: 7669–7674.
Jin RJ, Kwak C, Lee SG, Lee CH, Soo CG, Park MS et al. The application of an anti-angiogenic gene (thrombospondin-1) in the treatment of human prostate cancer xenografts. Cancer Gene Ther 2000; 7: 1537–1542.
Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J . Inhibition of angiogenesis in vivo by interleukin-12. J Natl Cancer Inst 1995; 87: 581–586.
Bikfalvi A, Gimenez-Gallego G . The control of angiogenesis and tumor invasion by platelet factor-4 and platelet factor-4-derived molecules. Semin Thromb Hemost 2004; 30: 137–144.
Feldman AL, Friedl J, Lans TE, Libutti SK, Lorang D, Miller MS et al. Retroviral gene transfer of interferon-inducible protein 10 inhibits growth of human melanoma xenografts. Int J Cancer 2002; 99: 149–153.
Addison CL, Arenberg DA, Morris SB, Xue YY, Burdick MD, Mulligan MS et al. The CXC chemokine, monokine induced by interferon-γ, inhibits non-small cell lung carcinoma tumor growth and metastasis. Hum Gene Ther 2000; 11: 247–261.
Angiolillo AL, Sgadari C, Tosato G . A role for the interferon-inducible protein 10 in inhibition of angiogenesis by interleukin-12. Ann NY Acad Sci 1996; 795: 158–167.
Soto H, Wang W, Strieter RM, Copeland NG, Gilbert DJ, Jenkins NA et al. The CC chemokine 6-Ckine binds the CXC chemokine receptor CXCR3. Proc Natl Acad Sci USA 1998; 95: 8205–8210.
Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I et al. Chemokine receptor specific for IP-10 and MIG: structure, function, and expression in activated T-lymphocytes. J Exp Med 1996; 184: 963–969.
Farber JM . Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol 1997; 61: 246–257.
Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG et al. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res 2000; 60: 1388–1393.
Bello L, Carrabba G, Giussani C, Lucini V, Cerutti F, Scaglione F et al. Low-dose chemotherapy combined with an anti-angiogenic drug reduces human glioma growth in vivo. Cancer Res 2001; 61: 7501–7506.
Hou JM, Liu JY, Yang L, Zhao X, Tian L, Ding ZY et al. Combination of low-dose gemcitabine and recombinant quail vascular endothelial growth factor receptor-2 as a vaccine induces synergistic antitumor activities. Oncology 2005; 69: 81–87.
Folkman J . Angiogenesis and apoptosis. Semin Cancer Biol 2003; 13: 159–167.
Yao B, He QM, Tian L, Xiao F, Jiang Y, Zhang R et al. Enhanced antitumor effect of the combination of tumstatin gene therapy and gemcitabine in murine models. Hum Gene Ther 2005; 16: 1075–1086.
Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R et al. Expression of the angiogenic factors vascular endothelial growth factor, acidic and basic fibroblast growth factor, tumor growth factor-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 1997; 57: 963–969.
Ruehlmann JM, Xiang R, Niethammer AG, Ba Y, Pertl U, Dolman CS et al. MIG (CXCL9) chemokine gene therapy combines with antibody-cytokine fusion protein to suppress growth and dissemination of murine colon carcinoma. Cancer Res 2001; 61: 8498–8503.
Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V . Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res 2003; 63: 8408–8413.
Wei YQ, Huang MJ, Yang L, Zhao X, Tian L, Lu Y et al. Immunogene therapy of tumors with vaccine based on xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci USA 2001; 98: 11545–11550.
Xiao F, Wei Y, Yang L, Zhao X, Tian L, Ding Z et al. A gene therapy for cancer based on the angiogenesis inhibitor, vasostatin. Gene Therapy 2002; 9: 1207–1213.
McAllister F, Steele C, Zheng M, Young E, Shellito JE, Marrero L et al. T cytotoxic-1 CD8+ T Cells are effector cells against pneumocystis in mice. J Immunol 2004; 172: 1132–1138.
Tan GH, Tian L, Wei YQ, Zhao X, Li J, Wu Y et al. Combinaton of low-dose cisplatin and recombination xenogeheic endoglin as a vaccin induses synergistic antitumor activities. Int J Cancer 2004; 112: 701–706.
Moorsel CJ, Pinedo HM, Smid K, Comijn EM, Voorn DA, Veerman G et al. Schedule-dependent pharmacodynamic effects of gemcitabine and cisplatin in mice bearing Lewis lung murine non-small cell lung tumours. Eur J Cancer 2000; 36: 2420–2429.
He QM, Wei YQ, Tian L, Zhao X, Su JM, Yang L et al. Inhibition of tumor growth with a vaccine based on xenogeneic homologous fibroblast growth factor receptor-1 in mice. J Biol Chem 2003; 278: 21831–21836.
Hoffmann J, Schirner M, Menrad A, Schneider MR . A highly sensitive model for quantification of in vivo tumor angiogenesis induced by alginate-encapsulated tumor cells. Cancer Res 1997; 57: 3847–3851.
Wei YQ, Wang QR, Zhao X, Yang L, Tian L, Lu Y et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat Med 2000; 6: 1160–1166.
Wild R, Ramakrishnan S, Sedgewick J, Griffioen AW . Quantitative assessment of angiogenesis and tumor vessel architecture by computer-assisted digital image analysis: effects of VEGF-toxin conjugate on tumor microvessel density. Microvasc Res 2000; 59: 368–376.
Fields RC, Shimizu K, Mule JJ . Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA 1998; 95: 9482–9487.
Lu Y, Wei YQ, Tian L, Zhao X, Yang L, Hu B et al. Immunogene therapy of tumors with vaccine based on xenogeneic epidermal growth factor receptor1. J Immunol 2003; 170: 3162–3170.
Acknowledgements
This work was sponsored by grants from National Key Basic Research Program of China (2004CB518800 and 2001CB510001), Project of National Natural Science Foundation of China, National 863 projects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, R., Tian, L., Chen, LJ. et al. Combination of MIG (CXCL9) chemokine gene therapy with low-dose cisplatin improves therapeutic efficacy against murine carcinoma. Gene Ther 13, 1263–1271 (2006). https://doi.org/10.1038/sj.gt.3302756
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302756
Keywords
This article is cited by
-
Engineering tumor-specific gene nanomedicine to recruit and activate T cells for enhanced immunotherapy
Nature Communications (2023)
-
Delivery of CXCL9/10/11 plasmid DNAs promotes the tumor-infiltration of T cells and synergizes with PD1 antibody for treating lung cancer
Cancer Nanotechnology (2022)
-
Correlative serum biomarker analyses in the phase 2 trial of lenvatinib-plus-everolimus in patients with metastatic renal cell carcinoma
British Journal of Cancer (2021)
-
Effect of memantine hydrochloride on cisplatin-induced neurobehavioral toxicity in mice
Acta Neurologica Belgica (2020)
-
Myeloid-restricted ablation of Shp2 restrains melanoma growth by amplifying the reciprocal promotion of CXCL9 and IFN-γ production in tumor microenvironment
Oncogene (2018)