Abstract
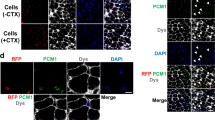
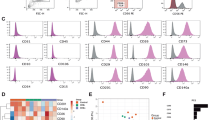
Facioscapulohumeral muscular dystrophy (FSHD) is an autosomal dominant disease characterized by a typical regional distribution, featuring composed patterns of clinically affected and unaffected muscles. No treatment is available for this condition, in which the pathophysiological mechanism is still unknown. Autologous transfer of myoblasts from unaffected to affected territories could be considered as a potential strategy to delay or stop muscle degeneration. To evaluate the feasibility of this concept, we explored and compared the growth and differentiation characteristics of myoblasts prepared from phenotypically unaffected muscles of five FSHD patients and 10 control donors. According to a clinically approved procedure, 109 cells of a high degree of purity were obtained within 16–23 days. More than 80% of these cells were myoblasts, as demonstrated by labeling of the muscle markers CD56 and desmin. FSHD myoblasts presented a doubling time equivalent to that of control cells; they kept high proliferation ability and did not show early telomere shortening. In vitro, these cells were able to differentiate and to express muscle-specific antigens. In vivo, they participated to muscle structures when injected into immunodeficient mice. These data suggest that myoblasts expanded from unaffected FSHD muscles may be suitable tools in view of autologous cell transplantation clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lunt P . Facioscapulohumeral muscular dystrophy: diagnostic and molecular aspects. In: Deymeer F (ed). Neuromuscular Diseases: From Basic Mechanisms to Clinical Management. Monographs in Clinical Neuroscience, Vol. 18. Karger: Basel, Freiburg, Paris, London, New York, New Delhi, Bangkok, Singapore, Tokyo, Sydney, 2000: 44–60.
Wijmenga C et al. Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat Genet 1992; 2: 26–33.
Hewitt JE et al. Analysis of the tandem repeat locus D4Z4 associated with facioscapulohumeral muscular dystrophy. Hum Mol Genet 1994; 3: 1287–1295.
Lunt PW et al. Correlation between fragment size at D4F104S1 and age at onset or at wheelchair use, with a possible generational effect, accounts for much phenotypic variation in 4q35-facioscapulohumeral muscular dystrophy (FSHD). Hum Mol Genet 1995; 4: 951–958.
Tawil R et al. Evidence for anticipation and association of deletion size with severity in facioscapulohumeral muscular dystrophy. Ann Neurol 1996; 39: 744–748.
Gabellini D, Green MR, Tupler R . Inappropriate gene activation in FSHD: a repressor complex binds a chromosomal repeat deleted in dystrophic muscle. Cell 2002; 110: 339–348.
Winokur ST et al. Expression profiling of FSHD muscle supports a defect in specific stages of myogenic differentiation. Hum Mol Gen 2003; 12: 2895–2907.
van der Kooi EL et al. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology 2004; 63: 702–708.
Skuk D, Roy B, Goulet M, Tremblay JP . Successful myoblast transplantation in primates depends on appropriate cell delivery and induction of regeneration in the host muscle. Exp Neurol 1999; 155: 22–30.
Skuk D, Goulet M, Roy B, Tremblay JP . Efficacy of myoblast transplantation in nonhuman primates following simple intramuscular cell injections: toward defining strategies applicable to humans. Exp Neurol 2002; 175: 112–126.
Blau HM, Webster C, Pavlath GK . Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 1983; 80: 4856–4860.
Webster C, Blau HM . Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet 1990; 16: 557–565.
Wright WE . Myoblast senescence in muscular dystrophy. Exp Cell Res 1985; 157: 343–354.
Irintchev A et al. Functional improvement of damaged adult mouse muscle by implantation of primary myoblasts. J Physiol 1997; 500: 775–785.
Arcila ME et al. Mass and functional capacity of regenerating muscle is enhanced by myoblast transfer. J Neurobiol 1997; 33: 185–198.
Huard J et al. High efficiency of muscle regeneration after human myoblast clone transplantation in SCID mice. J Clin Invest 1994; 93: 586–599.
Kinoshita I et al. Very efficient myoblast allotransplantation in mice under FK506 immunosuppression. Muscle Nerve 1994; 17: 1407–1415.
Kinoshita I, Vilquin JT, Roy R, Tremblay JP . Successive injections in mdx mice of myoblasts grown with bFGF. Neuromuscul Disord 1996; 6: 187–193.
Partridge TA et al. Conversion of mdx myofibres from dystrophin-negative to dystrophin-positive by injection of normal myoblasts. Nature 1989; 337: 176–179.
Morgan JE, Pagel CN, Sherratt T, Partridge TA . Long-term persistence and migration of myogenic cells injected into pre-irradiated muscles of mdx mice. J Neurol Sci 1993; 115: 191–200.
Gussoni E et al. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature 1992; 356: 435–438.
Huard J et al. Human myoblast transplantation: preliminary results of 4 cases. Muscle Nerve 1992; 15: 550–560.
Karpati G et al. Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol 1993; 34: 8–17.
Law PK et al. Dystrophin production induced by myoblast transfer therapy in Duchenne muscular dystrophy. Lancet 1990; 336: 114–115.
Mendell JR et al. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N Engl J Med 1995; 333: 832–838.
Miller RG et al. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve 1997; 20: 469–478.
Morandi L et al. Lack of mRNA and dystrophin expression in DMD patients three months after myoblast transfer. Neuromuscul Disord 1995; 5: 291–295.
Neumeyer AM et al. Pilot study of myoblast transfer in the treatment of Becker muscular dystrophy. Neurology 1998; 51: 589–592.
Tremblay JP et al. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant 1993; 2: 99–112.
Skuk D, Goulet M, Roy B, Tremblay JP . Myoblast transplantation in whole muscle of non human primates. J Neuropathol Exp Neurol 2000; 59: 197–206.
Skuk D et al. Dystrophin expression in myofibers of Duchenne muscular dystrophy patients following intramuscular injections of normal myogenic cells. Mol Therapy 2004; 9: 475–482.
Hagege AA et al. Viability and differentiation of autologous skeletal myoblast grafts in ischaemic cardiomyopathy. Lancet 2003; 361: 491–492.
Menasché P et al. First successful clinical myoblast transplantation for heart failure. Lancet 2001; 357: 279–280.
Menasche P et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 2003; 41: 1078–1083.
Pagani FD et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. J Am Coll Cardiol 2003; 41: 879–888.
Pouly J et al. Does the functional efficacy of skeletal myoblast transplantation extend to nonischemic cardiomyopathy? Circulation 2004; 110: 1626–1631.
Vignos PJ, Spencer GE, Archibald KC . Management of progressive muscular dystrophy in childhood. JAMA 1963; 184: 89–96.
Illa I, Leon-Monzon M, Dalakas MC . Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol 1992; 31: 46–52.
Lanier LL, Testi R, Bindl J, Phillips JH . Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med 1989; 169: 2233–2238.
Webster C et al. Isolation of human myoblasts with the fluorescence-activated cell sorter. Exp Cell Res 1988; 174: 252–265.
Li ZL, Lilienbaum A, Butler-Browne G, Paulin D . Human desmin-coding gene: complete nucleotide sequence, characterization and regulation of expression during myogenesis and development. Gene 1989; 78: 243–254.
Allsopp RC et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA 1992; 89: 10114–10118.
Decary S et al. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther 1997; 8: 1429–1438.
Decary S et al. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord 2000; 10: 113–120.
van der Maarel SM et al. De novo facioscapulohumeral muscular dystrophy: frequent somatic mosaicism, sex-dependent phenotype, and the role of mitotic transchromosomal repeat interaction between chromosomes 4 and 10. Am J Hum Genet 2000; 66: 26–35.
Goldman JP et al. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol 1998; 103: 335–342.
Cooper RN et al. A new immunodeficient mouse model for human myoblast transplantation. Hum Gene Therapy 2001; 12: 823–831.
Skuk D et al. Transplantation of human myoblasts in SCID mice as a potential muscular model for myotonic dystrophy. J Neuropathol Exp Neurol 1999; 58: 921–931.
Vilquin JT et al. Myoblast transplantations lead to the expression of the laminin alpha 2 chain in normal and dystrophic (dy/dy) mouse muscles. Gene Therapy 1999; 6: 792–800.
Winokur ST et al. Facioscapulohumeral muscular dystrophy (FSHD) myoblasts demonstrate increased susceptibility to oxidative stress. Neuromuscul Disord 2003; 13: 322–333.
Blau HM et al. Differentiation properties of pure populations of human dystrophic muscle cells. Exp Cell Res 1983; 144: 495–503.
Furling D et al. Defective satellite cells in congenital myotonic dystrophy. Hum Mol Genet 2001; 10: 2079–2087.
Renault V et al. Regenerative potential of human skeletal muscle during aging. Aging Cell 2002; 1: 132–139.
Vandebrouck C et al. Normal calcium homeostasis in dystrophin-expressing facioscapulohumeral muscular dystrophy myotubes. Neuromuscul Disord 2002; 12: 266–272.
Hoffman EP, Brown Jr RH, Kunkel LM . Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987; 51: 919–928.
Xu H et al. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc Natl Acad Sci USA 1994; 91: 5572–5576.
Xu H, Wu XR, Wewer UM, Engvall E . Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nat Genet 1994; 8: 297–302.
Richard I et al. Loss of calpain 3 proteolytic activity leads to muscular dystrophy and to apoptosis-associated IkappaBalpha/nuclear factor kappaB pathway perturbation in mice. J Cell Biol 2000; 151: 1583–1590.
Acknowledgements
This work was supported by a grant from the ‘Association Française contre les Myopathies’ (AFM). This study has been promoted by the CHU de Nice. The production of control myoblasts was supported by both AFM and the ‘Assistance Publique-Hôpitaux de Paris’ (France). We thank Dr James Di Santo (Institut Pasteur, Paris) for the kind gift of the immunodeficient Rag mice, Dr Marc Jeanpierre for FSHD molecular diagnosis, Dr Vincent Mouly for telomere evaluation, Drs Gillian Buttler-Browne and Marc Fiszman for critical review of the manuscript, Drs Philippe Chaumet-Riffaud and Solange Solbes-Latourette for helpful assistance at setting this preclinical protocol and Miss Jessica Shama for helpful revision of the document. We thank the company Myosix SA and Mr Frédéric Chereau for giving access to the myoblast culture methodology. Finally, we would also like to thank all the patients for their participation to this study.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vilquin, JT., Marolleau, JP., Sacconi, S. et al. Normal growth and regenerating ability of myoblasts from unaffected muscles of facioscapulohumeral muscular dystrophy patients. Gene Ther 12, 1651–1662 (2005). https://doi.org/10.1038/sj.gt.3302565
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302565
Keywords
This article is cited by
-
Macrophages and the maintenance of homeostasis
Cellular & Molecular Immunology (2021)
-
In vivo stem cell tracking using scintigraphy in a canine model of DMD
Scientific Reports (2020)
-
Skeletal muscle cell transplantation: models and methods
Journal of Muscle Research and Cell Motility (2020)
-
Isolation and Characterization of Human Myoblast Culture In Vitro for Technologies of Cell and Gene Therapy of Skeletal Muscle Pathologies
Bulletin of Experimental Biology and Medicine (2018)
-
Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle
Cell Death & Disease (2015)