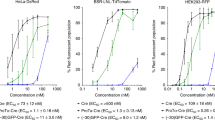
Abstract
Recently, a novel cationic polymer, dextran–spermine (D–SPM) was developed for gene delivery. An efficient transfection was obtained using this polycation for a variety of genes and cell lines in serum-free or serum-poor medium. However, transfection using the water-soluble D–SPM-based polyplexes decreased with increasing serum concentration in cell culture in a concentration-dependent manner, reaching 95% inhibition at 50% serum in the cell growth medium. In order to overcome this obstacle, oleyl derivatives of D–SPM (which form micelles in aqueous phase) were synthesized at 1, 10, and 20 mol% of oleyl moiety to polymer ɛ-NH2 to form N-oleyl-D–SPM (ODS). Polyplexes based on ODS transfected well in medium containing 50% serum. Comparison with polyplexes based on well-established polymers (branched and linear polyethyleneimine) and with DOTAP/Cholesterol lipoplexes showed that regarding β-galactosidase transgene expression level and cytotoxicity in tissue culture, the D–SPM and ODS compare well with the above polyplexes and lipoplexes. Intracellular trafficking using FITC-labeled ODS and Rhodamine-labeled pGeneGrip plasmid cloned with hBMP2 monitored by confocal microscopy revealed that during the transfection process the fluorescent-labeled polymer concentrates in the Golgi apparatus and around the nucleus, while the cell cytoplasm was free of fluorescent particles, suggesting that the polyplexes move in the cell toward the nucleus by vesicular transport through the cytoplasm and not by a random diffusion. We found that the plasmids penetrate the cell nucleus without the polymer. Preliminary results in zebra fish and mice demonstrate the potential of ODS to serve as an efficient nonviral vector for in vivo transfection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ye S, Cole-Strauss AC, Frank B, Kmiec EB . Targeted gene correction: a new strategy for molecular medicine. Mol Med Today 1998; 4: 431–437.
Shuey DJ, McCallus DE, Giordano T . RNAi: gene-silencing in therapeutic intervention. Drug Discov Today 2002; 7: 1040–1046.
Brunner S et al. Overcoming the nuclear barrier: cell cycle independent nonviral gene transfer with linear polyethylenimine or electroporation. Mol Ther 2002; 5: 80–86.
Nishikawa M, Huang L . Nonviral vectors in the new millennium: delivery barriers in gene transfer. Hum Gene Ther 2001; 12: 861–870.
Dani SU . The challenge of vector development in gene therapy. Braz J Med Biol Res 1999; 32: 133–145.
Rubanyi GM . The future of human gene therapy. Mol Aspects Med 2001; 22: 113–142.
Han S, Mahato RI, Sung YK, Kim SW . Development of biomaterials for gene therapy. Mol Ther 2000; 2: 302–317.
Templeton NS . Liposomal delivery of nucleic acids in vivo. DNA Cell Biol 2002; 21: 857–867.
Putnam D, Gentry CA, Pack DW, Langer R . Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc Natl Acad Sci USA 2001; 98: 1200–1205.
Kabanov AV . Taking polycation gene delivery systems from in vitro to in vivo. Pharm Sci Technol Today 1999; 2: 365–372.
Boussif O et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 1995; 92: 7297–7301.
Benns JM et al. pH-sensitive cationic polymer gene delivery vehicle: N-Ac-poly(L-histidine)-graft-poly(L-lysine) comb-shaped polymer. Bioconjug Chem 2000; 11: 637–645.
Bielinska AU et al. Application of membrane-based dendrimer/DNA complexes for solid phase transfection in vitro and in vivo. Biomaterials 2000; 21: 877–887.
Azzam T et al. Polysaccharide-oligoamine based conjugates for gene delivery. J Med Chem 2002; 45: 1817–1824.
Azzam T et al. Cationic polysaccharides for gene delivery. Macromolecules 2002; 35: 9947–9953.
Azzam T, Eliyahu H, Makovitzki A, Domb AJ . Dextran–spermine conjugate: an efficient vector for gene delivery. Macromol Symp 2003; 195: 247–261.
Azzam T, Eliyahu H, Makovitzki A, Domb AJ . Hydrophobized dextran–spermine conjugates as potential vector for in vitro gene transfection. J Control Release 2004; 96: 309–323.
Priev A, Zalipsky S, Cohen R, Barenholz Y . Determination of critical micelle concentration of lipopolymers and other amphiphiles: comparison of sound velocity and fluorescent measurements. Langmuir 2002; 18: 612–617.
Shinitzky M, Barenholz Y . Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta 1978; 515: 367–394.
Eliyahu et al. Relationships between chemical composition, physical properties and transfection efficiency of polysaccharide–spermine conjugates (submitted for publication).
Bergeron RJ et al. Polyamine analogue regulation of NMDA MK-801 binding: a structure–activity study. J Med Chem 1996; 39: 5257–5266.
Eliyahu H, Servel N, Domb AJ, Barenholz Y . Lipoplex-induced hemagglutination: potential involvement in intravenous gene delivery. Gene Therapy 2002; 9: 850–858.
Zhou XH, Klibanov AL, Huang L . Lipophilic polylysines mediate efficient DNA transfection in mammalian cells. Biochim Biophys Acta 1991; 1065: 8–14.
Lewis JG et al. A serum-resistant cytofectin for cellular delivery of antisense oligodeoxynucleotides and plasmid DNA. Proc Natl Acad Sci USA 1996; 93: 3176–3181.
Eliyahu H et al. Biodistribution and transgene expression of dextran–spermine polyplexes and DOTAP/Cholesterol lipoplexes administered locally and systemically: effect of physicochemical properties and stability of complexes in serum (submitted for publication).
Xu Y, Szoka Jr FC . Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 1996; 35: 5616–5623.
Wattiaux R, Laurent N, Wattiaux-De Coninck S, Jadot M . Endosomes, lysosomes: their implication in gene transfer. Adv Drug Deliv Rev 2000; 41: 201–208.
Luo D, Saltzman WM . Synthetic DNA delivery systems. Nat Biotechnol 2000; 18: 33–37.
Pouton CW, Seymour LW . Key issues in non-viral gene delivery. Adv Drug Deliv Rev 2001; 46: 187–203.
Wightman L et al. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J Gene Med 2001; 3: 362–372.
Davis ME . Non-viral gene delivery systems. Curr Opin Biotechnol 2002; 13: 128–131.
Godbey WT, Wu KK, Mikos AG . Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci USA 1999; 96: 5177–5181.
Simberg D, Hirsch-Lerner D, Nissim R, Barenholz Y . Comparison of different commercially-available cationic lipid-based transfection kits. J Liposome Res 2000; 10: 1–13.
Barenholz Y, Amselem S . Liposome preparation and related techniques. In: Gregoriadis G (ed). Liposome Technology, Vol. 1, 2nd edn. CRC Press: Boca Raton, FL, 1993, pp 527–616.
Simberg D et al. The role of organ vascularization and lipoplex-serum initial contact in intravenous murine lipofection. J Biol Chem 2003; 278: 39858–39865.
Zuidam NJ, Hirsch-Lerner D, Margulies S, Barenholz Y . Lamellarity of cationic liposomes and mode of preparation of lipoplexes affect transfection efficiency. Biochim Biophys Acta 1999; 1419: 207–220.
Simberg D, Weisman S, Talmon Y, Barenholz Y . DOTAP (and other cationic lipids): chemistry, biophysics, and transfection. Crit Rev Ther Drug Carr Syst 2004; 21: 257–317.
Honigman A et al. Imaging transgene expression in live animals. Mol Ther 2001; 4: 239–249.
Acknowledgements
This study was partially supported by the AFIRST, French–Israel Cooperation on Gene Therapy, and by the US–Israel Binational Fund (BSF) to AD, and by the Barenholz Fund and the Israel Science Foundation (ISF) to YB. We would like to thank Dr M Tarshish and Dr Y Zilberman for technical assistance and Mr S Geller for editing this manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eliyahu, H., Makovitzki, A., Azzam, T. et al. Novel dextran–spermine conjugates as transfecting agents: comparing water-soluble and micellar polymers. Gene Ther 12, 494–503 (2005). https://doi.org/10.1038/sj.gt.3302395
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302395
Keywords
This article is cited by
-
Star-Shaped Tetraspermine Enhances Cellular Uptake and Cytotoxicity of T-Oligo in Prostate Cancer Cells
Pharmaceutical Research (2015)
-
Biodegradable poly(amine-co-ester) terpolymers for targeted gene delivery
Nature Materials (2012)
-
RETRACTED ARTICLE: Engineered Polyallylamine Nanoparticles for Efficient In Vitro Transfection
Pharmaceutical Research (2007)