Abstract
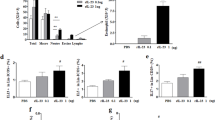
Allergic asthma is characterized by airway hyper-responsiveness (AHR) and cellular infiltration of the airway with predominantly eosinophils and Th2 cells. The normal resolution of inflammation in the lung occurs through the regulated removal of unneeded cells by Fas–Fas ligand-mediated apoptosis. Fas ligand (FasL) is a member of the tumor necrosis factor family, and when bound to Fas, it induces apoptosis of the cells. To examine the effect of the FasL gene on airway inflammation and immune effector cells in allergic asthma, recombinant adenovirus expressing murine FasL (Ad-FasL) was delivered intratracheally into ovalbumin (OVA)-immunized mice. We found that a single administration of Ad-FasL in OVA-immunized mice significantly alleviated AHR and eosinophilia by inducing the apoptosis of eosinophils and/or reducing eosinophil attractant factors, such as IL-5 and eotaxin levels. The number of infiltrated lymphocytes and Th2 cytokines, including IL-5 and IL-13, decreased in OVA-immunized mice by administration of Ad-FasL. KC and TNF-α production also decreased in Ad-FasL-treated OVA-immunized mice. These findings indicated that the administration of Ad-FasL to OVA-sensitized mice significantly suppressed pulmonary allergic responses. Although more studies are needed, these results suggested that Ad-FasL might be applied as an alternative therapy for allergic asthma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Herrick CA, Bottomly K . To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol 2003; 3: 405–412.
Leong KP, Huston DP . Understanding the pathogenesis of allergic asthma using mouse models. Ann Allergy Asthma Immunol 2001; 87: 96–109.
Larche M, Robinson DS, Kay AB . The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 2003; 111: 450–463.
Umetsu DT et al. Asthma: an epidemic of dysregulated immunity. Nat Immunol 2002; 3: 715–720.
Wiktor-Jedrzejczak W . Eosinophil development. Immunol Today 1993; 14: 238.
Hamelmann E et al. Anti-interleukin 5 but not anti-IgE prevents airway inflammation and airway hyperresponsiveness. Am J Respir Crit Care Med 1999; 160: 934–941.
Cieslewicz G et al. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J Clin Invest 1999; 104: 301–308.
Shen HH et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol 2003; 170: 3296–3305.
Tomkinson A et al. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am J Respir Crit Care Med 2001; 163: 721–730.
Kuperman DA et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002; 8: 885–889.
Li L et al. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J Immunol 1999; 162: 2477–2487.
Pope SM et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol 2001; 108: 594–601.
Mattes J et al. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med 2002; 195: 1433–1444.
Wilder JA et al. Dissociation of airway hyperresponsiveness from immunoglobulin E and airway eosinophilia in a murine model of allergic asthma. Am J Respir Cell Mol Biol 1999; 20: 1326–1334.
Leckie MJ et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000; 356: 2144–2148.
Flood-Page PT et al. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med 2003; 167: 199–204.
Osborne BA . Apoptosis and the maintenance of homoeostasis in the immune system. Curr Opin Immunol 1996; 8: 245–254.
Maher S, Toomey D, Condron C, Bouchier-Hayes D . Activation-induced cell death: the controversial role of Fas and Fas ligand in immune privilege and tumour counterattack. Immunol Cell Biol 2002; 80: 131–137.
Brunner T et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 1995; 373: 441–444.
Dhein J et al. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 1995; 373: 438–441.
Ju ST et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 1995; 373: 444–448.
Haslett C . Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med 1999; 160: S5–S11.
Druilhe A et al. Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol 1998; 19: 747–757.
Vignola AM et al. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol 1999; 103: 563–573.
Suzuki M et al. Immune responses against replication-deficient adenovirus inhibit ovalbumin-specific allergic reactions in mice. Hum Gene Ther 2000; 11: 827–838.
Behera AK, Kumar M, Lockey RF, Mohapatra SS . Adenovirus-mediated interferon gamma gene therapy for allergic asthma: involvement of interleukin 12 and STAT4 signaling. Hum Gene Ther 2002; 13: 1697–1709.
Walter DM et al. IL-18 gene transfer by adenovirus prevents the development of and reverses established allergen-induced airway hyperreactivity. J Immunol 2001; 166: 6392–6398.
Jose PJ et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med 1994; 179: 881–887.
Rothenberg ME, Luster AD, Leder P . Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in interleukin 4-induced tumor suppression. Proc Natl Acad Sci USA 1995; 92: 8960–8964.
Lukacs NW et al. TNF-α mediates recruitment of neutrophils and eosinophils during airway inflammation. J Immunol 1995; 154: 5411–5417.
Lee J et al. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol 1995; 155: 2158–2164.
Tsuyuki S et al. Activation of the Fas receptor on lung eosinophils leads to apoptosis and the resolution of eosinophilic inflammation of the airways. J Clin Invest 1995; 96: 2924–2931.
Gochuico BR et al. Airway epithelial Fas ligand expression: potential role in modulating bronchial inflammation. Am J Physiol-Lung Cell Mol Physiol 1998; 274: L444–L449.
Woolley KL et al. Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am J Respir Crit Care Med 1996; 154: 237–243.
Ohta K et al. In vivo effects of apoptosis in asthma examined by a murine model. Int Arch Allergy Immunol 2001; 124: 259–261.
Doucet C et al. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. J Clin Invest 1998; 101: 2129–2139.
Zhu Z et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiology abnormalities, and eotaxin production. J Clin Invest 1999; 103: 779–788.
Hagimoto N et al. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol 1997; 17: 272–278.
Matute-Bello G et al. Recombinant human Fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am J Physiol Lung Cell Mol Physiol 2001; 281: L328–L335.
Matute-Bello G et al. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol 2001; 158: 153–161.
Ogasawara J et al. Lethal effect of the anti-Fas antibody in mice. Nature 1993; 364: 806–809.
Muruve DA et al. Adenovirus-mediated expression of Fas ligand induced hepatic apoptosis after systemic administration and apoptosis of ex vivo-infected pancreatic islet allografts and isografts. Hum Gene Ther 1997; 8: 955–963.
He TC et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514.
Stampfli MR et al. Adenoviral infection inhibits allergic airways inflammation in mice. Clin Exp Allergy 1998; 28: 1581–1590.
Miwa K et al. Capase 1-independent IL-1β release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med 1998; 4: 1287–1292.
Chen JJ, Sun Y, Nabel GJ . Regulation of the proinflammatory effects of Fas Ligand (CD95L). Science 1998; 282: 1714–1717.
Bruder JT et al. Improved production of adenovirus vectors expressing apoptotic transgenes. Hum Gene Ther 2000; 11: 139–149.
Suen JL, Chuang YH, Chiang BL . In vitro tolerance breakdown with dendritic cells pulsed with U1A protein in non-autoimmune mice: the induction of a high level of autoantibodies but not renal pathological changes. Immunology 2002; 106: 326–335.
Nicoletti I et al. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 1991; 139: 271–279.
Hamelmann E et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 1997; 156: 766–775.
Lee YL et al. Construction of single-chain interleukin-12 DNA plasmid to treat airway hyperresponsiveness in an animal model of asthma. Hum Gene Ther 2001; 12: 2065–2079.
Chiang DJ et al. Ribavirin or CpG DNA sequence-modulated dendritic cells decrease the IgE level and airway inflammation. Am J Respir Crit Care Med 2003; 168: 575–580.
Acknowledgements
This work was supported by a grant from the National Science Council of the Republic of China.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chuang, YH., Fu, CL., Lo, YC. et al. Adenovirus expressing Fas ligand gene decreases airway hyper-responsiveness and eosinophilia in a murine model of asthma. Gene Ther 11, 1497–1505 (2004). https://doi.org/10.1038/sj.gt.3302325
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302325
Keywords
This article is cited by
-
Small Interfering RNA Targeting T-cell Ig Mucin-3 Decreases Allergic Airway Inflammation and Hyperresponsiveness
Inflammation (2013)
-
Arsenic trioxide alleviates airway hyperresponsiveness and eosinophilia in a murine model of asthma
Cellular & Molecular Immunology (2010)
-
Small interfering RNA against interleukin-5 decreases airway eosinophilia and hyper-responsiveness
Gene Therapy (2008)
-
Fas-ligand-expressing adenovirus-transfected dendritic cells decrease allergen-specific T cells and airway inflammation in a murine model of asthma
Journal of Molecular Medicine (2006)
-
Anti-Fas mAb-induced apoptosis and cytolysis of airway tissue eosinophils aggravates rather than resolves established inflammation
Respiratory Research (2005)