Abstract
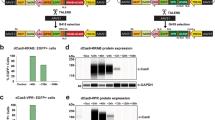
The expression of genes from genomic loci can be relatively complex, utilizing exonic, intronic and flanking sequences to regulate tissue and developmental specificity. Infectious bacterial artificial chromosomes (iBACs) have been shown to deliver and express large genomic loci (up to 135 kb) into primary cells for functional analyses. The delivery of large genomic DNA inserts allows the expression of complex loci and of multiple splice variants. Herein, we demonstrate for the first time that an iBAC will deliver and correctly express in human glioma cells the entire CDKN2A/CDKN2B genomic region, which encodes for at least three important cell-cycle regulatory proteins (p16INK4a, p14ARF and p15INK4b). Two of these proteins are expressed from overlapping genes, utilizing alternative splicing and promoter usage. The delivered locus expresses each gene at physiological levels and cellular responses (apoptosis versus growth arrest) occur dependent on cellular p53 status, as expected. The work further demonstrates the potential of the iBAC system for the delivery of genomic loci whose expression is mediated by complex splicing and promoter usage both for gene therapy applications and functional genomics studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sorek R, Armitai M . Piecing together the significance of splicing. Nat Biotechnol 2001; 19: 196.
Sharpless NE, DePinho RA . The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev 1999; 9: 22–30.
Conrad C, Vianna C, Freeman M, Davies P . A polymorphic gene nested within an intron of the tau gene: implications for Alzheimer's disease. Proc Natl Acad Sci USA 2002; 99: 7751–7756.
Tufarelli C et al. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat Genet 2003; 32: 157–165.
Soubrier F et al. High-resolution genetic mapping of the ACE-linked QTL influencing circulating ACE activity. Eur J Hum Genet 2002; 10: 553–561.
Galas DJ . Making sense of the sequence. Science 2001; 291: 1257–1260.
Wolfsberg TG, McEntyre J, Schuler GD . Guide to the draft human genome. Nature 2001; 409: 824–826.
Modrek B, Lee C . A genomic view of alternative splicing. Nat Genet 2002; 30: 13–19.
Thomas CE, Ehrhardt A, Kay MA . Progress and problems with the use of viral vector for gene therapy. Nat Rev Genet 2003; 4: 346–358.
Wade-Martins R et al. An infectious transfer and expression system for genomic DNA loci in human and mouse cells. Nat Biotechnol 2001; 19: 1067–1070.
Wade-Martins R, Saeki Y, Chiocca EA . Infectious delivery of a 135 kb LDLR genomic locus leads to regulated complementation of low-density lipoprotein receptor deficiency in human cells. Mol Ther 2003; 7: 604–612.
Saeki Y et al. Improved helper virus-free packaging system for HSV amplicon vectors using an ICP27-deleted, oversized HSV-1 DNA in a bacterial artificial chromosome. Mol Ther 2001; 3: 591–601.
Sherr CJ . The INK4a/ARF network in tumour supression. Nature Rev Mol Cell Biol 2001; 2: 731–737.
Quelle DE, Zindy F, Ashmun RA, Sherr CJ . Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 1995; 83: 993–1000.
Bates S et al. p14ARF links the tumour supressors RB and p53. Nature 1998; 395: 124–125.
Weber JD et al. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol 1999; 1: 20–26.
Robertson KD, Jones PA . Tissue-specific alternative splicing in the human INK4a/ARF cell cycle regulatory locus. Oncogene 1999; 18: 3810–3820.
Tsubari M, Tiihonen E, Laiho M . Cloning and characterization of p10, an alternatively spliced form of p15 cyclin-dependent kinase inhibiton. Cancer Res 1997; 57: 2966–2973.
Simon M et al. Alternative splicing of the p15 cdk inhibitor in glioblastoma multiforme. Acta Neuropathol 2001; 102: 167–174.
Caldas C et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet 1994; 8: 27–32.
Schmidt EE, Ichimura K, Reifenberger G, Collins VP . CDKN2(p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res 1994; 54: 6321–6324.
Moulton T et al. MTS1/p16/CDKN2 lesions in primary glioblastoma multiforme. Am J Pathol 1995; 146: 613–619.
Simon M, Koster G, Menon AG, Schramm J . Functional evidence for a role of combined CDKN2A (p16-p14ARF)/CDKN2B (p15) gene inactivation in malignant gliomas. Acta Neuropathol 1999; 98: 444–452.
Nakamura M et al. p14ARF deletion and methylation in genetic pathways to glioblastomas. Brain Pathol 2001; 11: 159–168.
Bachoo RM et al. Epidermal growth factor receptor and Ink4a/Arf: Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 2002; 1: 269–277.
Uhrbom L et al. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res 2002; 62: 5551–5558.
Borén J et al. A simple and efficient method for making site-directed mutants, deletions, and fusions of large DNA such as P1 and BAC clones. Genome Res 1996; 6: 1123–1130.
Simon M, Koster G, Menon AG, Schramm J . Functional evidence for a role of combined CDKN2A (p16-p14ARF))/CDKN2B (p15) gene inactivation in malignant gliomas. Acta Neuropathol 1999; 98: 444–452.
Geraghty RJ et al. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 1998; 280: 1618–1620.
Wade-Martins R et al. Stable correction of a genetic deficiency in human cells by an episome carrying a 115 kb genomic transgene. Nat Biotechnol 2000; 18: 1311–1314.
Diller L et al. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol 1990; 10: 5772–5781.
Komata T et al. Combination therapy of malignant glioma cells with 2-5A antisense telomerase RNA and recombinant adenovirus p53. Gene Therapy 2000; 7: 2071–2079.
Schiedner G et al. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat Genet 1998; 18: 180–183.
Stoll SM et al. Epstein-Barr virus/human vector provides high-level, long-term expression of a1-antitrypsin in mice. Molecular Therapy 2001; 4: 122–129.
Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY . Primary brain tumours in adults. Lancet 2003; 361: 323–331.
Mischel PS, Cloughesy TF . Targeted molecular therapy of GBM. Brain Pathol 2003; 13: 52–61.
Dunn IF, Black PM . The neurosurgeon as local oncologist: cellular and molecular neurosurgery in malignant glioma therapy. Neurosurgery 2003; 52: 1411–1424.
Kleihues P, Ohgaki H . Primary and secondary glioblastoma: from concept to clinical diagnosis. Neuro-Oncology 1999; 1: 44–51.
Kleihues P, Ohgaki H . Phenotype versus genotype in the evolution of astrocytic brain tumors. Toxicol Pathol 2000; 28: 164–170.
Sandig V et al. Adenovirally transferred p16IN4/CDKN2 and p53 genes cooperate to induce apoptotic tumor cell death. Nat Med 1997; 3: 313–319.
Schreiber M, Muller WJ, Singh G, Graham FL . Comparison of the effectiveness of adenovirus vectors expressing cyclin kinase inhibitors p16INK4A, p18INK4C, p19INK4D, p21WAF1/CIP1 and p27KIP1 in inducing cell cycle arrest, apoptosis and inhibition of tumorigenicity. Oncogene 1999; 18: 1663–1676.
Gao N et al. The exogenous wild-type p14ARF gene induces growth arrest and promotes radiosensitivity in human lung cancer cell lines. J Cancer Res Clin Oncol 2001; 127: 359–367.
Ghaneh P et al. Adenovirus-mediated transfer of p53 and p16INK4a results in pancreatic cancer regression in vitro and in vivo. Gene Therapy 2001; 8: 199–208.
Park Y-B et al. Alterations in the INK4a/ARF locus and their effects on the growth of human osteosarcoma cell lines. Cancer Genet Cytogenet 2002; 133: 105–111.
Yang CT et al. A comparison analysis of anti-tumor efficiency of adenoviral gene replacement therapy (p14ARF and p16INK4A) in human mesothelioma. Anticancer Res 2003; 23: 33–38.
Naruse I et al. High concentrations of recombinant adenovirus expressing p16 gene induces apoptosis in lung cancer cell lines. Anticancer Res 1998; 18: 4275–4282.
Baylin SB et al. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet 2001; 10: 687–692.
Wade-Martins R, Frampton J, James MR . Long-term stability of large insert genomic DNA episomal shuttle vectors in human cells. Nucleic Acids Res 1999; 27: 1674–1682.
Acknowledgements
We thank Dr David Louis (Massachusetts General Hospital, Charlestown, MA, USA) for providing samples from the Massachusetts General Hospital Brain Tumor Bank, for initially suggesting this experiment and for helpful discussion and advice. This work was supported by grants from The Wellcome Trust, the National Cancer Institute (CA692460) and the Berkowitz-Knott Fund for Brain Tumor Research to EAC. RW-M was a Wellcome Trust International Prize Travelling Research Fellow, and thanks the Trust for their continued support.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Inoue, R., Moghaddam, K., Ranasinghe, M. et al. Infectious delivery of the 132 kb CDKN2A/CDKN2B genomic DNA region results in correctly spliced gene expression and growth suppression in glioma cells. Gene Ther 11, 1195–1204 (2004). https://doi.org/10.1038/sj.gt.3302284
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302284
Keywords
This article is cited by
-
The infectious BAC genomic DNA expression library: a high capacity vector system for functional genomics
Scientific Reports (2016)
-
The potential application of a transcriptionally regulated oncolytic herpes simplex virus for human cancer therapy
British Journal of Cancer (2014)
-
DNA genome of spontaneously occurring deletion mutants of herpes simplex virus type 1 lacking one copy of the inverted repeat sequences of the L component
Archives of Virology (2011)
-
ICP0 Inhibits the Decrease of HSV Amplicon-mediated Transgene Expression
Molecular Therapy (2009)
-
Physiological Transgene Regulation and Functional Complementation of a Neurological Disease Gene Deficiency in Neurons
Molecular Therapy (2009)