Abstract
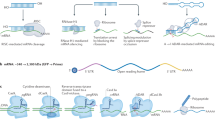
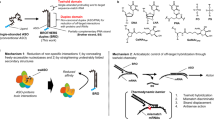
The design of potent systems for the delivery of charged and noncharged molecules that target genes of interest remains a challenge. We describe a novel technology that combines a new generation of peptide nucleic acids (PNAs), or HypNA-pPNAs, with a new noncovalent peptide-based delivery system, Pep-2, which promotes efficient delivery of PNAs into several cell lines. We have validated the potential of this technology by showing that Pep2-mediated delivery of an antisense HypNA-pPNA chimera directed specifically against cyclin B1 induces rapid and robust downregulation of its protein levels and efficiently blocks cell cycle progression of several cell lines, as well as proliferation of cells derived from a breast cancer. Pep-2-based delivery system was shown to be 100-fold more efficient in delivering HypNA-pPNAs than classical cationic lipid-based methods. Whereas Pep-2 is essential for improving the bioavailability of PNAs and HypNA-pPNAs, the latter contribute significantly to the efficiency and specificity of the biological response. We have found that Pep-2/HypNA-pPNA strategy promotes potent antisense effects, which are approximately 25-fold greater than with classical antisense oligonucleotide directed specifically against the same cyclin B1 target. Taken together, these data demonstrate that peptide-mediated delivery of HypNA-pPNAs constitutes a very promising technology for therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dias N, Stein CA . Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther 2002; 1: 347–355.
Dove A . Antisense and sensibility. Nat Biotechnol 2002; 20: 121–124.
Opalinska JB, Gewirtz AM . Nucleic-acid therapeutics: basis principles and recent application. Nat Rev Drug Discovery 2002; 1: 503–514.
Sazani P, Vacek MM, Kole R . Short-term and long-term modulation of gene expression by antisense therapeutics. Curr Opin Biotechnol 2002; 13: 468–472.
Kurrech J . Antisense technologies: improvement through novel chemical modifications. Eur J Biochem 2003; 270: 1628–1644.
Nielsen PE . Peptide nucleic acids as therapeutic agents. Curr Opin Struct Biol 1999; 9: 353–357.
Good L et al. Bactericidal antisense effects of peptide-PNA conjugates. Nat Biotechnol 2001; 19: 360–364.
Egholm M et al. PNA hybridizes to complementary oligonucleotides obeying the Watson–Crick hydrogen-bonding rules. Nature 1993; 365: 566–568.
Nielsen PE, Egholm M, Berg RH, Buchardt O . Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991; 254: 1497–1500.
Koppelhus U, Nielsen PE . Cellular delivery of peptide nucleic acid. Adv Drug Delivery Rev 2003; 55: 267–280.
Tyler BM et al. Peptide nucleic acids targeted to the neurotensin receptor and administered i.p. cross the blood–brain barrier and specifically reduce gene expression. Proc Natl Acad Sci USA 1999; 96: 7053–7058.
Aldrian-Herrada G et al. A peptide nucleic acid (PNA) is more rapidly internalized in cultured neurons when coupled to a retro-inverso delivery peptide. The antisense activity depresses the target mRNA and protein in magnocellular oxytocin neurons. Nucleic Acids Res 1998; 26: 4910–4916.
Rapozzi V et al. Antiproliferative effect in chronic myeloid leukaemia cells by antisense peptide nucleic acids. Nucleic Acids Res 2002; 30: 3712–3721.
Cutrona G et al. Effects in live cells of a c-myc anti-gene PNA linked to a nuclear localization signal. Nat Biotechnol 2000; 18: 300–302.
Stock RP et al. Inhibition of gene expression in Entamoeba histolytica with antisense peptide nucleic acid oligomers. Nat Biotechnol 2001; 19: 231–234.
Braun K et al. A biological transporter for the delivery of peptide nucleic acids (PNAs) to the nuclear compartment of living cells. J Mol Biol 2002; 318: 237–243.
Pooga M et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat Biotechnol 1998; 16: 857–861.
Koppelhus U et al. Cell-dependent differential cellular uptake of PNA, peptides, and PNA-peptide conjugates. Antisense Nucleic Acids Drug Dev 2002; 2: 51–63.
Branden LJ, Christensson B, Smith CI . In vivo nuclear delivery of oligonucleotides via hybridizing bifunctional peptides. Gene Therapy 2001; 8: 84–87.
Sazani P et al. Systemically delivered antisense oligomers upregulate gene expression in mouse tissues. Nat Biotechnol 2002; 12: 1228–1233.
Tackett A, Corey D, Raney K . Non-Watson–Crick interactions between PNA and DNA inhibit the ATPase activity of bacteriophage T4 Dda helicase. Nucleic Acids Res 2002; 30: 950–957.
Braasch D, Corey D . Synthesis, analysis, purification, and intracellular delivery of peptide nucleic acids. Methods 2001; 23: 97–107.
Weiler J et al. Hybridisation based DNA screening on peptide nucleic acid (PNA) oligomer arrays solid phase pythesis of directly linked PNA-RNA-hybrids. Nucleic Acids Res 1997; 25: 2792–2799.
Bergman F, Bannwarth W, Tam S . Solid phase synthesis of directly linked PNA-DNA hybrids. Tetrahedron Lett 1995; 36: 6823–6826.
Efimov VA et al. Detection of the 5′-cap structure of messenger RNAs with the use of the cap-jumping approach. Nucleic Acids Res 2001; 29: 4751–4759.
Efimov V et al. PNA-related oligonucleotide mimics and their evaluation for nucleic acid hybridization studies and analysis. Nucleosides Nucleotides Nucleic Acids 2001; 20: 419–428.
Efimov VA, Chakhmakhcheva OG . Conjugates of polyacrylamide with oligonucleotides and their mimetics for diagnostic purposes. Bioorg Khim 1999; 25: 848–854.
Morris MC et al. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol 2001; 19: 1173–1176.
Morgan DO . Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 1997; 13: 261–291.
Sausville EA . Complexities in the development of cyclin-dependent kinase inhibitor drugs. Trends Mol Med 2002; 8: 32–37.
Morris MC, Gondeau C, Tainer JA, Divita G . Kinetic mechanism of activation of the Cdk2/cyclin A complex. Key role of the C-lobe of the Cdk.. J Biol Chem. 2002; 277: 23847–23853.
Labbé JC et al. Purification of MPF from starfish: identification as the H1 histone kinase p34cdc2 and a possible mechanism for its periodic activation. Cell 1989; 57: 253–263.
Peter M et al. Initial activation of cyclin B1-cdc2 kinase requires phosphorylation of cyclin B1. EMBO Rep 2002; 3: 551–556.
Pichon C, Monsigny M, Roche AC . Intracellular localization of oligonucleotides: influence of fixative protocols. Antisense Nucleic Acids Drug Dev 1999; 9: 89–93.
Richard JP et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem 2003; 278: 585–590.
Mologni L, Marchesi E, Neilsen P, Gambacorti-Passerini C . Inhibition of promyelocytic leukemia (PML)/retinoic acid receptor-a and PML expression in acute promyelocytic leukemia cells by anti-PML peptide nucleic acid. Cancer Res 2001; 61: 5468–5473.
Zhang X, Ishihara T, Corey DR . Strand invasion by mixed base PNAs and a PNA-peptide chimera. Nucleic Acids Res 2000; 28: 3332–3338.
Nielsen PE, Egholm M, Buchardt O . Sequence-specific transcription arrest by peptide nucleic acid bound to the DNA template strand. Gene 1994; 149: 139–145.
Hanvey JC et al. Antisense and antigene properties of peptide nucleic acids. Science 1992; 258: 1481–1485.
Lohse J, Dahl O, Nielsen PE . Double duplex invasion by peptide nucleic acid: a general principle for sequence-specific targeting of double-stranded DNA. Proc Natl Acad Sci USA 1999; 96: 11804–11808.
Langel U (ed). Cell Penetrating Peptides: Processes and Application. Pharmacology & Toxicology Series. CRC Press: Boca Raton, FL, 2002.
Morris MC et al. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res 1997; 25: 2730–2736.
Kurreck J, Bieber B, Jahnel R, Erdmann VA . Comparative study of DNA enzymes and ribozymes against the same full-length messenger RNA of the Vanilloid Receptor subtype I. J Biol Chem 2002; 277: 7099–7107.
Acknowledgements
This work was supported in part by the Centre National de la Recherche Scientifique (CNRS) and by grants from the Agence Nationale de Recherche sur le SIDA (ANRS) European community (aLK2-cT-2001-01451) and the Association pour la Recherche sur le Cancer to MCM (ARC-4326) and to GD (ARC-5271). The Pep-1/Chariot project was supported by a grant from Active Motif (Carlsbad, CA, USA). We also thank M Dorée for continuous support and helpful discussions and P Travo for technical advice on microscopy.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morris, M., Chaloin, L., Choob, M. et al. Combination of a new generation of PNAs with a peptide-based carrier enables efficient targeting of cell cycle progression. Gene Ther 11, 757–764 (2004). https://doi.org/10.1038/sj.gt.3302235
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302235
Keywords
This article is cited by
-
Novel peptide (RATH) mediated delivery of peptide nucleic acids for antiviral interventions
Applied Microbiology and Biotechnology (2021)
-
Cell-penetrating Peptides as Versatile Vehicles for Oligonucleotide Delivery
Molecular Therapy (2012)
-
Fluorescent Biosensors of Intracellular Targets from Genetically Encoded Reporters to Modular Polypeptide Probes
Cell Biochemistry and Biophysics (2010)
-
Nuclear entry of nonviral vectors
Gene Therapy (2005)
-
PNA2/DNA Triplexes: Stability and Specificity
Russian Journal of Genetics (2005)